ETIOLOGY
Accommodative esotropia is precipitated by the following hereditary disorders: (1) hypermetropia; and/or (2) a high accommodative convergence to accommodation (AC/A) ratio. Patients may have both hypermetropia and a high AC/A ratio.
As Donders2 described, the first cause is the convergence associated with accommodation applied to clear the blurred retinal image caused by hypermetropia. If the retinal image is allowed to remain blurred, the hypermetropic patient is not accommodating and the eyes remain straight. However, clearing the blurred hypermetropic image is accomplished by accommodation, and there is also a synkinetic accommodative esodeviation.
A high AC/A ratio occurs in patients with normal refractions, but with an abnormal relationship between accommodation and its synkinetically associated accommodative convergence, which can cause an esodeviation at near. The amount of convergence associated with every 1 diopter (D) of accommodation may vary from slight to marked.
CHARACTERISTICS
In children, accommodative esophoria is usually asymptomatic. If a symptom appears, it is usually asthenopia, which occurs after prolonged near work. As the fusional divergence fatigues, controlling the esophoria by maintaining fusion becomes increasingly difficult. Eventually, esotropia momentarily replaces esophoria, and the patient experiences diplopia. The diplopia causes the patient to react by reducing the accommodation, hence lessening the associated accommodative convergence, which reduces the esophoria to the level at which the fatigued fusional divergence can regain fusion. Esotropia returns to esophoria, but the retinal image is blurred as a result of underaccommodation and, as a consequence, visual acuity is reduced. The reduced acuity stimulates the patient to increase the accommodation, which, in turn, causes the diplopia to return. This recurrent cycle of diplopia vacillating with blurred vision is often experienced by the fatigued esophoric patient. Patients with accommodative esophoria caused by a high AC/A ratio have these symptoms at near, whereas those with accommodative esophoria caused by hypermetropia have symptoms at both distance and near.
The onset of accommodative esotropia may occur at any age between 6 months and 7 years; the average age of the patient at onset is 2½ years, regardless of the cause. Apparently, this is the usual age at which the youngster first accommodates sufficiently to appreciate the visual gain, although diplopia is experienced. Accommodation is not sustained, but the recurring esotropia produces the clinical pattern of intermittent esotropia. Attention to fine visual detail causes momentary esotropia at both distance and near if hypermetropia is the cause, and only at near if a high AC/A ratio is the cause. Some patients maintain the intermittent esotropia pattern without manifestations of an increase toward constant esotropia; however, others increase the frequency and duration of esotropia and rapidly convert to constant esotropia.
CLINICAL INVESTIGATION
The clinical investigation of these patients demands an evaluation of the cycloplegic refraction, the AC/A ratio, and the fusional divergence amplitude because these three factors determine the cause of esodeviation and the patient's ability to contain the accommodative esodeviation and maintain fusion.
Cycloplegic Refraction
An adequate cycloplegic refraction need not be atropine refraction. One drop of 2% cyclopentolate (Cyclogyl) within 40 minutes provides within 0.25 D of the plus refractive error produced by one drop of 1% atropine three times daily for 3 days in white children. Deeply pigmented irides require more than one drop of 2% cyclopentolate: namely, the addition of one drop of 2.5% phenylephrine hydrochloride (Neo-Synephrine), and 40 minutes later one drop of 1% tropicamide (Mydriacyl). This produces superb cycloplegia 1 hour after the first drops are instilled in the most deeply pigmented eyes. Ideally, evaluation of the cycloplegic refraction should be included in the examination conducted during the initial appointment, and if needed, glasses should be prescribed at the conclusion of the examination. Also, regardless of the cycloplegic drugs used in children, a certain residual hypermetropia remains. Repeat cycloplegic refraction within weeks after prescribing the first glasses usually discloses more hypermetropia than was detected at the initial refraction.
Accommodative Convergence to Accommodation (AC/A) Ratio
The AC/A ratio is determined by comparing the distance prism, near prism, and alternate cover accommodation-controlled measurements. A near esodeviation measurement within 10Δ of the distance measurement is considered within the normal range. An excess of 10Δ difference between distance and near constitutes a high AC/A measurement. Since the severity of the high AC/A ratio varies from patient to patient, it is graded as follows:
Grade 1: The difference from 11Δ to 20Δ between the distance and near
measurements
Grade 2: The difference from 21Δ through 30Δ
Grade 3: The difference in excess of 30Δ
Infants and children fixate toys at distance and near as the prism and alternate cover measurements are performed. Children who submit to a visual acuity test with Snellen letters or the Snellen illiterate E fixate the same targets for their distance-near testing. The lines of columns of letters are read as the cover test is performed on literate children, and illiterate children point out the direction of the E, which are continuously presented during cover testing.
Any combination of refraction and AC/A ratio can be found, but statistically there is a definite relationship. Patients with a normal AC/A ratio have relatively more hypermetropia, and those with a high AC/A ratio have relatively less hypermetropia. One study3 revealed the relationships shown in Table 1.3 These findings are in accordance with expectations, since patients with a high AC/A ratio should have to accommodate less than patients with a normal AC/A ratio to produce equal angles of accommodative esodeviation.
TABLE 1. Relationship Between AC/A Ratio and Refraction in Patients with
Accommodative Esotropia
| Patients | AC/A | Refractions |
| 378 | High | + 2.25 |
| 289 | Normal | + 4.75 |
The evidence for altering the AC/A ratio after surgery is inconclusive. In a prospective study of 38 patients with concomitant strabismus, the effect of strabismus surgery was investigated.4 In both esotropia and exotropia, the AC/A ratio was found to decrease after surgery. A trend became evident, suggesting an increased risk of overcorrection in those children with esotropia with a high preoperative AC/A ratio equal to or greater than 7:1.
Fusional Divergence Amplitude
The fusional divergence amplitude usually ranges between 12Δ and 20Δ; when the accommodative esodeviations exceed this range, esotropia prevails. By trial and error, the fusional divergence amplitude can be determined indirectly by doing the prism and alternate cover test while the patient is wearing the minimal hypermetropic lenses that permit fusion. Patients with accommodative esodeviation who remain intermittently esotropic for several years tend to maintain the largest fusional divergence amplitudes. The patient who lapses into constant esotropia soon loses the large fusional divergence amplitude previously possessed when the strabismus was intermittent.
Sensory and Motor Complications
Sensory and motor complications soon evolve if esotropia repeatedly vacillates with esophoria. Initially, diplopia is experienced when esotropia first appears; however, the young child soon learns suppression and adopts anomalous retinal correspondence (ARC) peripheral fusion, removing any sensory annoyances that occur during the esotropic phase. Until this happens, the child often manifests diplopia and visual confusion by expressing it verbally, by closing or covering one eye, and by awkwardness. As soon as these sensory annoyances are eliminated by development of the sensorial adaptations, the patient is happier and more willing to tolerate the tropia. Eventually, constant esotropia may replace intermittent esotropia. After developing suppression and ARC and while still intermittently esotropic, these patients—when their eyes are straight—have normal retinal correspondence (NRC) and central and peripheral fusion. Thus, these patients instantly adjust their sensory status to conform to the alignment of the eyes.
A motor complication also occurs in intermittent esotropia; it is presumably a change in the medial rectus muscles secondary to their increased and more frequent contraction. Whatever the change—hypertrophy or contracture—a gradual increase in nonaccommodative esodeviation appears. Eventually, the nonaccommodative esodeviation buildup exceeds the fusional divergence amplitude in most patients, and the intermittent esotropia is replaced by constant esotropia. As the angle of esotropia increases, the ARC values and localization of the suppression scotoma change accordingly to conform to the larger angle.
With constant esotropia comes an opportunity for amblyopia to develop. In contrast to those with congenital esotropia, most patients with acquired esotropia select one eye for fixation to the exclusion of the other eye; this soon results in amblyopia of the unused eye. If, by chance, alternate fixation is chosen, amblyopia is prevented. Amblyopia is unrelated to suppression and ARC. Although the intermittent phase of the accommodative esotropia prevails, suppression and ARC may begin; in some patients, however, amblyopia does not develop because there is sufficient bifoveal fixation to prevent it. Only when constant esotropia replaces intermittent esotropia is the strabismus capable of causing amblyopia to develop in the patient with nonalternate fixation. However, suppression and ARC are always present in constant acquired esotropia, regardless of alternate or nonalternate fixation; they also occur during the intermittent esotropia phase.
TREATMENT
Early recognition of the disorder and early initiation of treatment are mandatory if the sensory and motor complications are to be prevented. The basic treatment involves some technique that curbs accommodation. This is accomplished either by an optical method (e.g., glasses) or by instillation of a parasympathomimetic drug (miotic) into the eyes.
The treatment varies according to the patient's age at the time of initial ophthalmologic consultation. For convenience, the treatment is described for three age groups: younger than 4 years, 4 to 8 years, and older than 8 years.
Children Younger Than 4 Years of Age
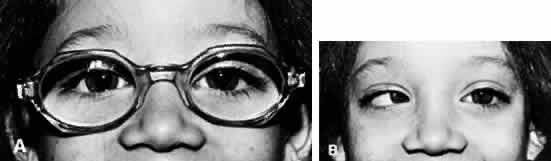
In all children younger than 4 years of age, the full cycloplegic refraction spectacle correction is prescribed (Fig. 1). Occasionally, the patient's history is not helpful, the findings are questionable, and the ophthalmologist wishes to gain evidence regarding improvement in the angle of esotropia by curbing the accommodation, but may have reason to doubt whether glasses are necessary. Alternatively, glasses may be rejected despite all efforts to encourage eventual acceptance, such as prescribing instillation of 1% atropine each morning for 1 month. If glasses cannot be used, it is helpful to prescribe instillation of a miotic each morning. The miotic should be considered temporary therapy: it is to be used to determine the antiaccommodation effect, or until the child has matured and accepts the needed glasses. Miotics should not be considered as alternative permanent antiaccommodation therapy equivalent to glasses.
|
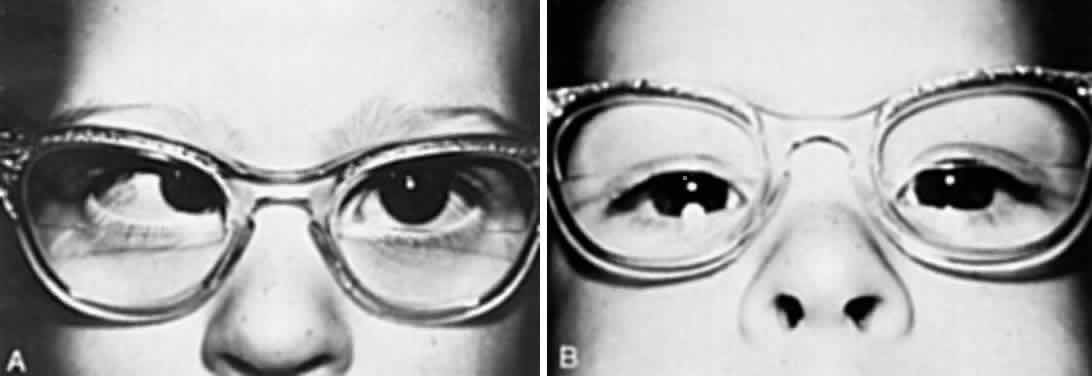
SPECTACLE CORRECTION. The patient is reexamined at monthly intervals until the ophthalmologist is certain that the glasses are controlling the accommodative esotropia. If the esotropia remains at near but the eyes are straight at distance, an additional + 2.5 D bifocal segment is prescribed (Fig. 2). Care is taken to inform the optician that the top of the lower segment must be higher for children than for adults. Ideally, the top of the segment must be 3 mm above the 6-o'clock limbus position. Segments that are too low are either viewed over, or the child must raise the chin too high in order to depress the eyes to see through the segments. If the optician places the top of the segment too high, the bifocals will be higher than midpupil, causing the chin to be depressed while viewing at distance through the upper portion of the bifocals. Children who do not readily accept the lower segment for near viewing and continue to cross their eyes for near viewing while looking through the upper portions of the lenses are started on 1% atropine instillation daily. This therapy is continued for up to 1 month. After the atropine is discontinued, the child usually continues to use the lower segment correctly. The ophthalmologist judges the correct acceptance of the lower segment by observing whether or not the child invariably raises the chin and depresses the eyes to look through the lower segment when small target material is presented at eye level or slightly above at a distance of 0.33 meter. The most satisfactory bifocal is a flat-top segment that traverses the entire width of the lens, such as the executive bifocal. With this type of lens, regardless of the position in which the child depresses the eyes in straight down gaze, left and down gaze, or right and down gaze, the lower segment is simultaneously engaged by each eye. The parents should make sure the glasses are in proper adjustment, since tilted or crooked glasses cause one eye to look through the lower bifocal segment and the other eye to look above it. Some patients obviously need bifocals, and these are prescribed as were the initial glasses. For example, the child who, without glasses, has straight eyes for distance but 35Δ of esotropia for near and ±1 D cycloplegic refractive error needs bifocals. Progressive addition lenses, such as the Varilux 2 lenses (Essilor Canada, Ltd., Ville Saint-Laurent, PQ) have been advocated because of the lack of image jump without the demarcation line, and because of the cosmetic improvement of the lenses without lines.5 There is difficulty, however, in determining the proper height of the progressive zone, and the lenses are more expensive.
Substituting a miotic for bifocals when the patient needs glasses because of a refractive error usually does not succeed, since the child tends to peer over the glasses and abandons them. Miotics work best in lieu of glasses, not in conjunction with them.
MIOTICS. Two miotics have been used extensively in North America for accommodative esotropia: isoflurophate (Floropryl 0.025% ointment)6–8 and echothiophate iodide (Phospholine Iodide 0.03% to 0.25%). These are long-acting cholinesterase inhibitors that facilitate neuromuscular transmission.9 Isoflurophate is inactivated by water and once was dispensed in USP anhydrous peanut oil. Currently it is not available in the United States. If control of the esodeviation is achieved during this time, the frequency of administration is diminished to every other day for 2 months and finally to twice a week (and in rare cases, once a week) for as long as therapy is required. Echothiophate is stable in a water solution if refrigerated; four concentrations are available (0.03%, 0.06%, 0.125%, and 0.25%). One drop is instilled in each eye each morning. Both drugs cause miosis, but more severe miosis is caused by isoflurophate than by echothiophate. Sustained miosis is often associated with pigmented hypertrophic epithelial cysts at the pupillary border. These cysts project into the pupillary space and may seal the space shut if they are allowed to develop further by continuing the medication. This occurs more readily with isoflurophate than with echothiophate. After discontinuing the miotic, the cysts diminish in size but remain for years as an obvious pigmented hyperplastic mass (“tags”) on the pupillary border as viewed in the slit lamp. The cysts can be prevented by instilling 2.5% phenylephrine hydrochloride twice daily.10 This diminishes the miosis slightly, but does not interfere with the function of potentiating the transmission of acetylcholine at the myoneural junction of the ciliary musculature. Miotics have a residual action for several days to a few weeks, making cycloplegia and mydriasis less than thorough during this period.
Although miotics have a residual action for several days to a few weeks, it appears to be unnecessary to discontinue echothiophate in order to obtain a reliable cycloplegic refraction. Raab11 compared the results of cycloplegic refraction in 18 patients on echothiophate, and then off echothiophate for 4 weeks. The average interval was 9 weeks (range, 2 to 39 weeks). The results were calculated to account for the various strengths of echothiophate (0.06%, 0.125%, or 0.25%), and for the interval off echothiophate. From the time that echothiophate was stopped, refraction was 4 weeks or less, the change in refraction was + 0.08 ± 0.48 D; at greater intervals, the change in refraction was -0.12 ± 0.29 D.
Although isoflurophate causes greater miosis, it performs better than echothiophate in controlling accommodative esodeviation. It cannot be titrated as easily, however, because of the single strength. Echothiophate has a systemic effect: it destroys the cholinesterase in plasma and erythrocytes. This constitutes an additional risk for the patient undergoing anesthesia, particularly if other anticholinesterase drugs, such as succinylcholine, are used during intubation to relax the pharyngeal muscles. As a result, the muscles used in respiration may be rendered functionless for a few hours, and the anesthesiologist may have to supply artificial ventilation for the patient until the muscles recover. Finally, subcapsular vacuoles, and even cataracts, have been reported in the eyes of patients receiving echothiophate. Neither drug can be considered innocuous, and the indication for their use must be evaluated carefully. No patient should receive either of these medications without being observed at least every 3 months. After the patient's deviation is controlled, either the concentration may be reduced or the frequency of instillation diminished progressively to every other day and then to every third day. The minimum dosage should control the deviation and allow fusion. The medication deteriorates with time, and the patient develops some degree of tolerance to it; these variables must also be considered. The medical approach to controlling accommodative esodeviation is more precarious and difficult to manage than the optical approach. Since accommodative esotropia requires long-term control, miotics are not the agents of choice for prolonged therapy. The prime importance of glasses in the management of accommodative esotropia has not been displaced by miotics.
CLINICAL COURSE. The cause of accommodative esotropia determines the clinical course. Accommodative esotropia due to hypermetropia and a normal AC/A ratio remains well controlled once glasses straighten the eyes. This is not true, however, for accommodative esotropia due to a high AC/A ratio. Despite the use of glasses to immediately control the esotropia, there is a significant possibility that a gradual, nonaccommodative esotropia component will appear. Table 2 shows that the more severe the high AC/A ratio, the greater the possibility of the patient's deteriorating and developing nonaccommodative esotropia, thus escaping the control that glasses rendered originally.12
TABLE 2. Relationship Between the Deterioration Rate and the Severity of
the High AC/A Ratio
| Patients | AC/A | Deterioration Rate (%) |
| 40 | Normal | 5 |
| 19 | Slightly high | 11 |
| 20 | Moderately high | 35 |
| 21 | Severely high | 43 |
In another study, Ludwig and associates13 analyzed the rate of deterioration in accommodative esotropia in relationship to the AC/A ratio. The AC/A relationships were graded according to the difference between the distance and near measurements:
Normal: 0Δ to 9Δ
Grade 1: 10Δ to 19Δ
Grade 2: 20Δ to 29 Δ
Grade 3: 30Δ and higher
Deterioration occurred in 7.7% of patients with a normal AC/A ratio, in 25% with grade 1 high AC/A ratio, 44% with grade 2 high AC/A ratio, and 52% with grade 3 high AC/A ratio, confirming that accommodative esotropia tends to deteriorate with greater frequency if the AC/A ratio is high.
Patients with a high AC/A ratio and a need for bifocals do not usually manifest improvement in the severity of their abnormal ratio until after 7 years of age. The hypermetropia disclosed by subsequent cycloplegic refraction usually increases until 6 years of age, levels off for 2 or more years, and after 8 years of age frequently decreases until the teens. Patients with accommodative esotropia controlled by glasses, including bifocals, are reexamined at 6-month intervals until 6 years of age and annually thereafter.
In a retrospective study, Wilson and colleagues14 reviewed the records of 127 patients with accommodative esotropia, who were within 10Δ of orthophoria, and who had stereopsis and other binocular sensory test measurements at their latest examination. With bifixation defined as 50 arc-seconds of stereopsis or better, 31 (24%) met this criterion; average follow-up was 89 months. Monofixation, or peripheral fusion, was present in the remaining 96 patients (76%); average follow-up was 84 months. In comparing the two groups, the patients with bifixation were less likely to have presented with constant esotropia (19% vs 39%), were more likely to be aligned within 8Δ of orthophoria with their first eyeglasses (84% vs 21%), were less likely to have worn bifocals (39% vs 59%), and less likely to have undergone surgery (23% vs 62%). Wilson and colleagues suggest that the maintenance of bifixation is possible in accommodative esotropia if the eyes can be straightened before the deviation becomes constant, or shortly thereafter. With the early therapy, amblyopia and deterioration of ocular alignment are less likely to occur. None of the patients with bifixation had constant esotropia lasting longer than 4 months.
Children who became constantly esotropic before receiving treatment and whose glasses did not reduce their angle to straight, or children whose angle was originally straight with glasses but escaped the control supplied by glasses and who are now esotropic with glasses, require surgery for the quantity of esotropia measured at distance with their full corrective lenses. Empirically, we have learned that patients with a high AC/A ratio who require surgery should receive more than the customary amount of surgery done for that angle of esotropia. For example, if the angle of deviation would normally require a 4-mm recession of the medial rectus muscles, we would do a 5-mm bilateral medial rectus recession for the patient with a high AC/A ratio. Any child on whom surgery is performed should have amblyopia eliminated by the appropriate occlusion therapy before the surgery. If, after wearing glasses for 1 month, the eyes remain esotropic and there is no amblyopia, surgery is performed. We have not noticed improvement in the angle of esotropia in patients wearing glasses for several months. The constantly esotropic eyes either straighten promptly with glasses or assume an angle of esotropia that remains approximately the same until surgery is performed.
A high rate of undercorrection has occurred when performing standard surgery for the nonaccommodative component, when full hypermetropic correction is worn.15–18 Because of these undercorrections, Wright and Bruce-Lyle18 have recommended augmented surgery for esotropia with high hypermetropia. In a retrospective study, they reviewed 70 patients with the following criteria: acquired esotropia, hypermetropia of 3 D or more, surgery for the residual esotropia with recession of the medial rectus muscle both eyes, full optical correction, surgery after 6 months of age, follow-up of at least 1 year, normal neurologic testing, and absence of eye muscle palsy. Based on the average of the deviation at distance and near, 30 patients received standard surgery; the other 40 patients had augmented surgery, based on the average of the near deviation, with and without correction. Correction to 10 D or less of orthophoria was achieved in 22 of the 30 patients (74%) in the standard surgery group; undercorrection was noted in the remaining 8 patients (26%). Correction to 10 D or less of orthophoria was accomplished in 35 of the 40 patients (88%) in the augmented surgery group; however, 10 D or more of consecutive exotropia developed in the remaining 5 patients (12%) with their full optical correction. After reduction of the hypermetropic power, or in some patients, removal of the eyeglasses, success was achieved in 39 of the 40 patients (98%). One patient had intermittent exotropia even after removing the eyeglasses.
Children 4 to 8 Years of Age
The treatment of accommodative esotropia in children between 4 and 8 years of age contrasts with that described for children younger than 4 years old in that the minimum power lenses required to maintain fusion and provide maximum visual acuity can be determined and prescribed. With accommodative targets at distance and near, as well as the cover-uncover test, the least power plus lenses that keep the eyes straight are prescribed, including bifocals if necessary. Ideally, esophoria is demonstrable by an alternate cover test with these minimal plus lenses, since it maintains a high degree of tone in the fusional divergence. This satisfies the objective of accommodative esotropia therapy, which is to reduce the esodeviation with an antiaccommodation measure just enough to allow accommodative esophoria to replace accommodative esotropia. The object is not to convert accommodative esotropia to orthophoria. Maintaining orthophoria with glasses over several years, never putting a load on the patient's fusional divergence, decreases the probability of eventual withdrawal of glasses without symptoms of asthenopia, blurred vision, and diplopia. It is generally impossible to apply this objective of therapy for accommodative esotropia to children younger than 4 years of age because the examiner is unable to evoke a response in this age group that is indicative of the visual acuity.
Children Older Than 8 Years of Age
In the treatment of accommodative esotropia in children older than 8 years of age, the ophthalmologist must realize that this is the earliest age at which improvement should be expected. The hypermetropia usually decreases, and the severity of the high AC/A ratio diminishes from this age into the early teens. The power of the spectacle plus lenses can usually be decreased and the bifocals reduced and frequently withdrawn. The prognosis for withdrawal of the spectacles must be related to the severity of the hypermetropia, the astigmatism, the anisometropia, and the AC/A ratio. Some patients who should have a good prognosis for part- or full-time spectacle withdrawal but show no spontaneous improvement may be induced to remove their glasses after expanding their fusional divergence amplitude, or at least encouraged to become more reliant on using their fusional divergence to its maximal potential. Patients are taught to limit their accommodation to that quantity that does not evoke more accommodative esodeviation than can be contained by the fusional divergence amplitude, even though the retinal image remains blurred, since suboptimal accommodation was applied to gain maximal visual acuity. Either an orthoptist or an ophthalmologist can teach this with the aid of diminishing-strength miotics.
The orthoptist teaches dissociation exercises to enable the patient with accommodative esotropia to remove the glasses. First, the patient is taught to maintain straight eyes without glasses in the unaccommodative state, accepting blurred vision. Next, small increments of accommodation that are associated with increasing amounts of esophoria are allowed. Repeating this maneuver increases the fusional divergence amplitude. Gradually, larger amounts of esodeviation are withstood until, eventually, clear vision and straight eyes are maintained. Physiologic diplopia (framing) and bar reading should be practiced during this routine to prevent the patient from lapsing into esotropia with suppression and to guarantee maintenance of fusion with clear vision. Training to increase the fusional divergence amplitude may be done at the same time by using gradually stronger base-in prisms while watching television and reading or by employing stereoscopic or Polaroid vectographic techniques (Orthofusor).
Miotics can be withdrawn gradually while preserving fusion. By gradually diminishing the concentration or frequency of the instillation of the miotic, the fusional divergence may be expanded. This requires the patient to accommodate more in order to obtain clear vision. This causes greater esophoria, which in turn puts more stress on the fusional divergence amplitude if binocularity is to be maintained. By gradually expanding the fusional divergence amplitude, some patients can eventually discontinue all medication, keeping the eyes straight and seeing well. The program ideally follows this plan:
- Remove glasses and start 0.125% echothiophate daily for 1 week.
- Decrease to 0.06% echothiophate daily for 1 week.
- Decrease to 0.06% echothiophate every other day for four doses.
- Decrease to 0.06% echothiophate every third day for three doses.
- Decrease to 0.06% echothiophate every fourth day for two doses.
- Decrease to 0.06% echothiophate every week for two doses.
- Discontinue all medication.
The patient is examined each time the dosage is changed, and further decrease is not made unless the eyes are straight. This routine can be combined with orthoptic dissociation exercises and fusional divergence amplitude exercises.
These procedures are successful in patients 8 years of age or older with moderate refractive errors and in those with moderately severe high AC/A ratios. If, however, the policy of providing no lens power above that necessary to keep the eyes straight is pursued from 4 years of age, most of those who required medication or orthoptics to rid themselves of glasses at 8 years of age will have achieved the same result by 10 to 12 years of age.
Deterioration of accommodative esotropia to nonaccommodative esotropia is a well-recognized complication requiring surgical intervention.19 Although most cases of accommodative esotropia subside by 10 to 12 years of age, there are many cases of accommodative esotropia, with or without deterioration, persisting into the later teen years and occasionally into adulthood.
Ludwig and colleagues20 studied 65 patients with accommodative esotropia who required bifocals to maintain near alignment. The average follow-up was 10.5 years. Forty patients (61.5%) were able to discontinue bifocals after an average of 5.5 years. Twenty-five patients (38.5%) continued to wear bifocals or reading glasses after an average follow-up of 9.7 years. Twenty patients in the first group (50%) and 9 patients in the second group (36%) required surgery for deterioration of accommodative esotropia. The average age of bifocal discontinuation was 9.3 years in the nonsurgical patients and 9.7 years in the surgical patients. The surgical patients had lower hyperopia (average, + 2.4 D) than the nonsurgical group (+ 3.5 D) and an earlier age of onset of bifocal wear (3.3 vs 4.6 years). Although bifocals may be discontinued successfully in a majority of patients at an average age of 9.5 years, a significant percentage require long-term wear, even among those who have had surgery. The only predictive factor for long-term bifocal wear was a relatively high AC/A ratio.