EXOPHORIA
If exophoria is asymptomatic, it requires no treatment. Symptoms are rare in young children and tend to occur with increasing frequency in patients older than 10 years of age.
Fusional convergence training by orthoptics is successful in most affected patients and should be the first method used to alleviate the patient's recurrent symptoms.26 The objective is to enhance the fusional convergence amplitude by various techniques. Treatment is emphasized for distance if the exodeviation is greater at distance than at near; near exercise is emphasized if the exodeviation is greater at near than at distance. While the patient does the exercise, the ophthalmologist should control the accommodation so that the fusional convergence is stimulated rather than the accommodative convergence. Small, detailed, distance targets are fixated for the distance exercises, and small print is read for the near exercise. Various combinations of loose 2PD, 3PD, 10PD, and 20PD prisms can be arranged horizontally to offer the following stepwise increments in stimulus to fusional convergence: 2PD, 3PD, 5PD, 7PD, 8PD, 10PD, 12PD, 13PD, 15PD, 17PD, 18PD, 20PD, 22PD, 23PD, 25PD, 27PD, 28PD, 30PD, 32PD, 33PD, and 35PD. The loose prism fusional convergence technique used in combination with a Polaroid screen fitted over the television while the patient wears Polaroid spectacle analyzers ensures that the patient is fusing at distance rather than suppressing as the base-out prism increments are made. Bar-reading is an excellent method of ensuring against suppression at near as the increments of base-out prisms are made. Bar-reading is accomplished by holding a narrow septum vertically midway between the eyes and the page; this obscures a different portion of the page for the right and left eye, but the entire page is seen undisturbed as long as the patient fuses.
Various stereoscopic techniques are also available for stimulating fusional convergence. One technique involves using a series of stereocards in a Wheatstone stereoscope; increasing convergence is demanded as the patient progresses through the series. Another method is built around the Polaroid vectographic technique: a series of Polaroid vectographic plates are viewed through Polaroid analyzers. As the patient proceeds through the series of plates, progressively more convergence is required to obtain the perception of stereopsis.
Whenever possible, the ophthalmologist should use accommodative convergence to compensate for exophoria in the subpresbyopic patient by undercorrecting hypermetropia and overcorrecting myopia. Forcing 2- to 3-D accommodation by appropriate spectacle power is usually well tolerated in young patients, but in older patients this therapy may provoke symptoms of asthenopia similar to those it seeks to alleviate, and the benefit is thus nil. In a series of 35 children with intermittent exotropia who wore overcorrecting minus lenses, Caltrider and Jampolsky27 reported a qualitative change, with poorly controlled intermittent exotropia improving to well-controlled exophoria in 46%. A quantitative decrease of at least 15 D and latency of the exodeviation while wearing overcorrecting minus lenses was found in 26%. There were no significant qualitative or quantitative changes in 28%. Minus lens therapy has also been advocated for control of postoperative exodeviations.28 In a series of 37 patients, preoperative diagnoses included 10 patients with esotropia and 27 with exotropia. Before treatment with minus lenses, over 75% had poor eye alignment, with manifest exotropia of 10 D or more, exophoria, or intermittent exotropia of 15 D or more. The over-minus therapy improved results to excellent in more than 75%, with intermittent exotropia of less than 10 D, or a exophoria of 7 to 14 D, classified as satisfactory. After a 2-year follow-up without therapy, 50% had excellent to satisfactory alignment and 33% had no to 6 D of intermittent exotropia. In addition, medical therapy is available to stimulate accommodative convergence. By instilling 2% homatropine eye drops, the AC/A ratio is increased, which provides more convergence to offset the exophoria. However, the symptoms of asthenopia and blurred vision that accompany this therapy regardless of the patient's age make it an impractical method for managing exophoria.
Base-in prism spectacles to compensate for the exophoria continue to have a place in the overall therapeutic regimen. Such spectacles are particularly useful in both presbyopic patients and subpresbyopic patients, regardless of age, who are unable to increase their fusional convergence amplitude with orthoptic training. In this connection, there is an established clinical entity that is ideally treated by base-in prism spectacles. It occurs in young people, is life-long, and causes symptoms during near vision manifested by blurred print and diplopia. Orthophoria or minimal exophoria is measured at distance, but a 12PD to 18PD exotropia is measured at near. Most significantly, the amplitude, or range, of accommodation is conspicuously less than the norm for the chronologic age. In addition, all fusional vergence amplitudes are obviously less than normal or almost nil, and, no matter how seriously the patient tries, these amplitudes cannot be expanded. This nearvision disorder presumably results from some deficiency in the optomotor reflexes. Accommodation, accommodative convergence, and fusional vergences are not there, and there is no way to improve them. Such patients should not receive prolonged orthoptic training. Presbyopic therapy with plus lens power is required for reading, along with base-in prisms to compensate for the near exotropia. The optic combination of these two therapies can be provided in reading spectacles; half-eye spectacles probably offer the best solution.
Surgery is seldom the answer for either distance or near exophoria, and it should be deferred until other methods of treatment have failed, unless the prism and alternate cover distance and near measurements are significant. Patients with either distance or near measurements that approach orthophoria are poor surgical candidates; an adult with approximately distant orthophoria and a symptomatic large angle of near exophoria is a particularly poor surgical risk. For such a patient with a low AC/A ratio, some ophthalmologists claim that resections of the medial rectus muscles improve the near exophoria without altering the preoperative distance orthophoria, but few have documented this claim of permanent improvement in near exophoria without a concomitant disturbing postoperative distance esotropia.
A study by von Noorden recommends resection of the medial rectus in persistent symptomatic convergence insufficiency but cautions that temporary consecutive esotropia occurs, requiring temporary prism control for some time after surgery.29
The exophoric patient with a large distance deviation and approximately near orthophoria, who never slips into intermittent exotropia while viewing at distance, is also almost never troubled with symptoms and rarely requires treatment of any kind, including surgery. The patient with a large angle of exophoria at both distance and at near (i.e., in excess of 12PD) and frequent symptoms does well with surgery, and it is justifiable to offer this treatment.
INTERMITTENT EXOTROPIA
Surgery is justified for intermittent exotropia if anisometropia or myopia has been adequately corrected with spectacles and if an eye continues to turn out intermittently. The patient has the best opportunity for a complete cure with elimination of the exoangle prior to the development of suppression and ARC. If the patient is 10 years of age or older and suppression and ARC are not present while the patient is exotropic, these sensorial adaptations to binocular vision will never develop, and the good prognosis for cure will continue unchanged. Until the patient reaches approximately 10 years of age, there is always the risk that suppression and ARC will be learned while exotropic; after these are learned, they can always be retained. These adaptations tend to be used and reused for any residual exodeviation, no matter how small, after surgery. Eventually, the small exodeviation tends to build back toward the preoperative levels. To prevent this discouraging result, the small residual postoperative exodeviation must be kept latent, as it is in patients without suppression and ARC. Attempts to prevent the suppression and ARC from ruining the immediate postoperative result by employing preoperative and postoperative orthoptics have usually failed.
Cooper and Leyman compared the results of surgical and orthoptic treatment of intermittent exotropia.30 Their 673 patients were divided into four categories of treatment: 1) occlusion only, 2) operation only, 3) orthoptic training and operation, and 4) orthoptic training only. Orthoptic exercises were of two types: antisuppression techniques and convergence training. Most of the group with only orthoptic training had exotropia less than 24PD, whereas most patients in the two surgical groups measured 25PD or more.
The highest percentage of good results, 59%, was in group 4, but this group also had smaller degrees of exotropia. Good results were obtained in 42% of group 2 and 52% of group 3. Cooper and Leyman encourage orthoptic training, when available, for small-angle exotropias and also to enhance the surgical results of exotropias above 25PD.
An opposing view was presented by Moore, who reviewed the orthoptic treatment of intermittent exotropia in 180 patients ranging in age from 3 to 18 years.31 None of these patients had good fusion on initial examination, 48 had fair fusion, and 132 had poor fusion. Treatment results were carefully recorded, and of the 57 patients in the surgery group, 33% were cured and 51% improved with a remaining intermittent deviation, for a total of 84% improved. Of the 106 patients receiving surgery plus orthoptic training, 30% were cured and 42% were improved with a remaining intermittent deviation, for a total improvement of 73%. Moore concluded that orthoptic training did not appear to increase the likelihood of a cure in intermittent exotropia.
Therefore, the most logical therapy for intermittent exotropia is surgical straightening of the eyes as soon as possible after a successful evaluation of the alignment is made and daily recurrence of the exotropia is documented.
In a study of lateral rectus recession, both eyes, for exotropia, Stoller and coworkers32 performed survival analysis, with survival time as the time from surgery to the time of failure after surgery. The estimated mean time to failure was 68 months. Surgical success was reported in 58% of cases, but outcome failures occurred in 42%, if 1) there was a distance exodeviation of more than 10PD at any time after surgery, 2) a distance esodeviation of more than 10PD more than 6 months postoperatively, or 3) further surgery was required for esotropia or exotropia. Successful postoperative alignment was more likely to occur with intermittent exotropia than with constant exotropia. Nonpredictors of success included: age at onset of exotropia, age at time of surgery, angle of deviation, early postoperative alignment, and the presence of amblyopia, anisometropia, incomitance, or other symptoms resulting from the exotropia.
Response to the prism adaptation test in 94 consecutive patients with intermittent exotropia has been reported by Ohtsuki and coworkers.33 Nonresponders included 43 patients whose adaptation to Fresnel prisms applied to both eyeglass lenses was 4PD or less, and the responders comprised 51 patients, whose deviation increased by 5 or more prism diopters. Pertinent findings included an increase in deviation with prism correction in patients whose initial deviation was less than 30PD and in patients younger than 7 years of age. Surgical results did not differ between the two groups at 1 and at 3 years. At 3 years postoperatively, the motor alignment, within 8PD of orthophoria, at 52%, and single binocular vision, at 83%, was higher in the prism-adapted group compared with that in nonresponders at 26% and 57%, respectively.
Pratt-Johnson and colleagues reported on the results of surgery on 100 patients with intermittent divergence-excess exotropia.34 Immediate postoperative results, measured on the first or second postoperative day, were compared with measurements a minimum of 1 year after surgery. Their strict criteria for cure included no tropia at any distance; near stereopsis of 40 seconds of arc; divergence amplitude of at least 5PD on the troposcope, with the immediate recognition of diplopia on exceeding the divergence amplitude; and good convergence amplitude, with total convergence and divergence amplitude exceeding 20PD.
Based on these criteria, 41 patients were cured. Of 59 patients who were not cured, 40 were within 10PD of orthophoria, producing a total of 81% of results that were cosmetically satisfactory.
The relationship between age at the time of surgery and final results was carefully studied. There were 39 patients younger than 4 years of age, with a median age of 2½ years, compared with 69 patients 4 years of age or older, with a median age of 5½ years. Of the patients younger than 4 years of age, 61% were cured, compared with 28% of the 4 years and older group, a statistically significant (p < .001) difference. Although the presence of a small vertical strabismus decreased the chances of obtaining a cure, the most significant factor in obtaining a cure was surgery before 4 years of age.
In a retrospective study of 111 consecutive patients with intermittent exotropia, Richard and Parks35 analyzed three groups, according to age at initial surgery. Group I comprised 41 patients younger than 3 years of age; group II, 42 patients aged 3 to younger than 6 years of age; and group III, 28 patients 6 years to younger than 17 years of age. Satisfactory results were defined as 10 D or less intermittent exotropia at distance, using prism and alternate cover testing. Using this definition, after initial surgery of recession of the lateral rectus of each eye, satisfactory results occurred in 61% of group I, 45% of group II, and 64% of group III. Unsatisfactory results were defined as undercorrection, a residual intermittent exotropia of more than 10 D, or overcorrection, consecutive esotropia. Results in group I were unsatisfactory in 39%; in group II, 55%; and in group III, 36%. Additional surgery was recommended for many patients with unsatisfactory initial surgical results. For those patients with at least a 2-year follow-up from the secondary surgery, a satisfactory result was achieved in 89% of group I, 86% of group II, and 100% of group III.
When combining all three groups, an initial satisfactory result was obtained in 56% of the patients after the initial recession of the lateral rectus of each eye. There were 38% undercorrections and 6% overcorrections. In the undercorrected group, additional surgery was necessary, producing 82% satisfactory results. In the overcorrected group, aggressive medical management, and subsequent recession of the medial rectus of each eye in almost all patients of this group, led to satisfactory results in 87%. Statistical analysis of the data failed to assign any significance to the age of the patient or the time intermittent exotropia was first noted and the time of the initial surgery, or of secondary surgery, and obtaining a satisfactory result. There also was no statistically significant difference demonstrated in obtaining a satisfactory surgical result if age groupings for delay in performing surgery were compared. Postponing the time of surgery did not improve the chance for obtaining a satisfactory result.
Intermittent exotropia causes visual symptoms in patients of all ages, unless suppression and ARC have developed. Elimination of the exoangle by surgery is justified in the absence of suppression and ARC simply on the basis of removing the recurrent symptoms and disregarding the merit of preventing the onset of suppression and ARC. The divergent eye is cosmetically disfiguring and always a source of embarrassment to the patient. Regardless of the patient's age and state of sensorial adaptations to the intermittent exotropia, surgery is justified to eliminate the visual symptoms and unusual appearance with which these patients present. In a series of 44 adults with intermittent exotropia ranging in age from 15 to 70, preoperative symptoms included diplopia, headaches, reading difficulty, ocular fatigue, and pain.36 Because of persistent diplopia with overcorrections, the authors recommended deliberate, slight undercorrections in adults, stating that a small, residual exotropia was more desirable than postoperative esotropia and diplopia. It is difficult to justify allowing these patients to remain untreated and to possibly deteriorate gradually from intermittent exotropia to constant exotropia, although not all ophthalmologists agree with this philosophy. Some claim that inasmuch as not all patients with intermittent exotropia manifest their exoangle as frequently when they are older as when younger, performing surgery on all young intermittent exotropes causes some patients to endure unnecessary surgery. This difference of opinion becomes an impossible argument because within the lifetime of one surgeon, all the facts necessary to support the surgeon's position cannot be accumulated. For example, even if the child improves to the extent that the intermittent exotropia occurs less than daily, it may reappear 20 years later with more frequency. This could be the source of the disorder in the rare patient with late onset who appears to develop intermittent exotropia in adulthood first. However, there is some merit in the argument for not operating on every child with intermittent exotropia, because some do spontaneously improve.7 Those with smaller exoangles are more inclined to improve spontaneously than those with larger angles. For this reason, surgery should be deferred in the intermittent exotropic child whose maximal prism and alternate cover distance measurement is less than 15PD, unless the exoangle increases or the intermittent exotropia increases in frequency and duration.
Part-time occlusion with early-onset unilateral intermittent or constant exotropia was reported in a series of 11 patients.37 The visual acuity was equal, and the nondeviating eye was occluded from 4 to 6 hours daily. All patients converted to heterophoria or orthophoria at least a portion of the time, and three became orthophoric without further patching. Three children eventually required surgery, on the average at 2½ years after onset of occlusion therapy. The technique of patching either postponed surgical intervention or converted exotropia to a long-term exophoria or orthophoria.
However, for all patients, regardless of age, whose distance exoangle can be reliably measured as 15PD or more, whose tropia recurs daily, and whose significant refractive error is compensated by spectacles, the ophthalmologist should give serious consideration to proceeding with surgery. Such surgery usually involves recession of the lateral rectus or recession of the lateral rectus muscle along with resection of the medial rectus muscle of the same eye. As an initial procedure, the improvement provided by resecting the medial rectus is usually not durable because the postoperative alignment gradually reverts to the preoperative status. Recession or resection of a single horizontal rectus muscle is not recommended because it produces asymmetry of lateral gaze and little or no change in the primary position alignment.
Nevertheless, several reports have recommended single lateral rectus muscle recession for small angle intermittent exotropia. Feretis and associates38 described minimal abduction deficiency for unilateral recession up to 12 mm when the intermittent exotropia ranged from 14 to 16 D. Deutsch and coworkers39 treated 30 patients with unilateral lateral rectus muscle recessions of 7.0 or 7.5 mm for exotropia of 15 to 20PD and found a mean effect of 17.6PD, stable to almost 24 months' follow-up. In a subsequent paper, Nelson and associates40 reported on 55 patients, with 15 to 20PD of exotropia, and unilateral lateral rectus recessions of 7.0, 7.5, or 8.0 mm. At their latest follow-up examination, 55 patients (51%) were orthophoric, but 24 patients (44%) were undercorrected, with 22 having residual exodeviations less than 8PD, and 3 patients (5%) were overcorrected. Weakley and Stager41 reported a success rate of 73% after unilateral lateral rectus muscle recessions of 6 to 10 mm with small- to moderate-angle intermittent or constant exotropia. Their success increased to 89% in patients under 4 years of age. One advantage reported was elimination of overcorrections, which can occasionally produce amblyopia or reduction of stereopsis in younger patients. Olitsky42 prospectively compared early (1-week) and late (6-month) postoperative alignment after unilateral lateral rectus recession for small to moderate intermittent exotropia. A satisfactory result, within 8PD, was achieved in 77% of patients at the 6-month examination. Olitsky concluded that there was not a significant difference in early versus late alignment with unilateral lateral lateral rectus surgery, that early overcorrection is not desirable, and that acceptable alignment in the first week provides the best prognosis for stability of postoperative alignment.
Unilateral surgery for exotropia with convergence weakness was studied prospectively by Kraft and coworkers.43 Unilateral recession of a lateral rectus and a resection or advancement of a medial rectus was performed, with the medial rectus strengthened more than the lateral rectus was recessed. The mean exotropia at distance was 18.3PD (range 8 to 35PD), and near exotropia was 30.1PD (range 16 to 50PD). The mean follow up was 7.9 months (range 6 to 26 months), with a mean distance deviation of 0.1PD (range 8PD for esotropia to 10PD for exotropia) and mean near deviation of 1.8PD (range 2PD for esotropia to 8PD for exotropia). The difference of near to distance deviation was reduced from a mean preoperative 11.8PD (range 8 to 21PD), to a mean postoperative 1.7PD (range 0 to 6PD). Their approach to surgery, basing the quantity of surgery on the near deviation, and strengthening the medial more than weakening the lateral rectus muscle, reduced the tendency to create large or long-term postoperative esodeviations. They stressed that this type of surgery should be restricted to patients who could not be controlled by nonsurgical therapy or those with large or poorly controlled exodeviations.
Choice of the initial surgical procedure used routinely by surgeons treating intermittent exotropia is rather evenly divided between recession of the lateral rectus and unilateral recession-resection of the horizontal rectus, with equally good results.44,45 It probably is not important, therefore, to argue the merits of either approach, because the overriding factor that dictates the surgeon's choice is confidence in the procedure. Recession of the lateral rectus is advocated by some surgeons because it is neat and symmetric. Some surgeons believe that recession-resection produces a more permanent result than recession alone because the resection of the medial rectus muscle “locks” the eye in better alignment, preserving the benefit of the recessed lateral rectus muscle. They also claim that the asymmetric horizontal measurements produced by the recession-resection are minor and that they gradually improve.
Botulinum treatment for strabismus was first reported in 1981 by Scott.46 A later paper by Scott and colleagues47 reported the results of Botulinum treatment of childhood strabismus. The frequency of correction to 10PD or less was only 45% in exotropia, with a follow-up period averaging 26 months after the last injection, with an average of 1.7 injections per patient. The results in all cases revealed that smaller deviations (10–20PD) were more frequently corrected, 73%, than larger deviations were (20–110PD), 54%. In a more recent paper, Spencer and associates48 reported on a nonrandomized, case-controlled study of patients with intermittent exotropia. Each patient received simultaneous bilateral lateral rectus injections of 2.5 units botulinum toxin type A, under anesthesia, and the mean preinjection deviation was reduced from 29 to 6PD. A satisfactory outcome was stable binocular alignment of orthophoria within 10PD, which was achieved in 69% (22 of 32). Only one set of bilateral injections was required in all patients between 24 and 56 months of age, irrespective of the initial exotropia angle, and was not associated with any secondary abnormalities.
Several factors determine the quantity of surgery used to correct the intermittent exotropia; however, there is still a baseline level of surgical intervention that must be performed according to the distance prism and alternate cover measurements. The baseline quantities are presented in Table 1. The table can be read in either of two ways. First, reading from top to bottom determines the quantity of surgery performed for exoangles ranging between 15PD and 30PD, and the millimeters of recession performed per prism diopter of exoangle as the initial procedure. It also shows the quantity of resections of the medial rectus muscles that are performed per prism diopter of exoangle as the secondary procedure. Second, by reading the table horizontally rather than vertically and from bottom to top rather than from top to bottom determines the millimeters of recession-resection of the horizontal rectus of one eye per prism diopter of exoangle. For 40PD, 50PD, and 60PD of exoangle, the surgeon selects an initial bilateral recession-resection procedure and performs the same quantity of surgery listed for 20PD, 25PD, and 30PD, respectively, on each eye.
TABLE 1. Quantity of Surgery Suggested According to Exoangle
| XT Angle | Recess LROU | Resect MROU |
| 15 | 4 mm | 3 mm |
| 20 | 5 mm | 4 mm |
| 25 | 6 mm | 5 mm |
| 30 | 7 mm | 6 mm |
| XT Angle | Recess LR | Resect MR |
LROU, lateral rectus muscle both eyes; MROU, medial rectus muscle both eyes; LR, lateral rectus; MR, medial rectus.
Berland and coworkers49 described 8 to 9 mm lateral rectus muscle surgery for deviations ranging from 35 to 65PD and reported surgical results, incidence of abduction deficits, and correlation with overcorrections. Only one operation was required in 80% of patients, but 20% required a second operation, two of three for overcorrection and one of three for undercorrection. Mild but persistent abduction deficits were found postoperatively in 30% but were not predictive of poor outcome. Concurrent oblique muscle surgery was associated with a higher risk of a poor result, with inferior oblique weakening associated with horizontal alignment undercorrection in every case reported. Superior oblique weakening was associated with one overcorrection and one undercorrection of the horizontal deviations.
Adjustments in the quantity of surgery performed per prism diopter of exoangle, as listed in Table 1, are required in patients with a low AC/A ratio and in those patients with lateral incomitance. The patient with a low AC/A ratio who manifests a near exoangle that exceeds the distance exoangle by 10PD or more requires 1 mm more surgery per muscle than the Table 1 indicates. The patient having 10PD or more reduction of exoangle on lateral gaze compared with the primary position requires 1 mm less surgery per muscle than Table 1 indicates.
The importance of lateral incomitance was first reported by Parks, who recommended decreasing the amount of recession ordinarily done for primary position exotropia when the measurements in right and left gaze are less than in the primary position.50 A small residual exophoria in the primary position and straight eyes in side gaze are preferable after surgery to orthophoria in the primary position and esotropia on side gaze. Moore subsequently suggested a disorder of divergence or convergence tonus as the mechanism of decreased exodeviation on lateral gaze.51 Concerning intermittent exotropia diminished on side gaze, she reported that 32% of patients experienced overcorrection, with esotropia increased on side gaze. In cases of intermittent exotropia without lateral incomitance, only 4% had overcorrection. These results occurred regardless of the type of intermittent exotropia or of the type of surgery performed. These results suggest that excessive surgery was performed in those patients with lateral incomitance.
Moore51 also reported an incidence of 22% of lateral incomitance in nonparetic exotropia. Repka and Arnoldi52 found lateral incomitance of more than 5 D at a much lower incidence, 9%. Thompson and Guyton53,54 have stressed holding the prism correctly for accuracy in measuring eye deviations. Repka and Arnoldi52 noted that holding the prisms incorrectly when measuring lateral gaze rotations causes the prism to have a different effective power. Further, they found that measurements of lateral gaze deviations produced smaller measurements when the prism was placed over the abducting eye in lateral incomitance, rather than over the adducting eye. Therefore, if the lateral gaze measurements are taken with a maximum 30 degree head turn, with the prism placed over the adducting eye, artifactitious incomitance produced by placing the prism over the abducting eye is minimized.55
Carlson and Jampolsky56 performed bilateral medial rectus and lateral rectus recessions on adjustable sutures in three patients, based on the hypothesis of tight medial rectus muscles in association with tight lateral rectus muscles as a cause of lateral incomitancy in intermittent exotropias. To compensate for the medial rectus recessions, the lateral rectus recessions exceeded the normal amount, and the primary position alignment with alleviation of lateral incomitancy was achieved. For marked lateral gaze incomitance in intermittent exotropia, medial rectus resections, with or without posterior fixation sutures, have been recommended.57 Those who received resections alone had small-angle esotropia persisting in lateral gaze, but those patients who also had posterior fixation sutures had straight eyes in all positions of gaze. There was no primary position near misalignment reported.
The surgeon who recesses the lateral rectus muscles should anticipate ± 20PD esotropia in the primary position on the second or third postoperative day.58,59 This is significantly reduced by the tenth postoperative day, and the immediate overcorrection usually disappears by the third postoperative week. The final result can be appraised by the sixth postoperative week.
Scott and coworkers60 agree that the best alignment 1 to 2 years after surgery occurs in the presence of a moderate overcorrection at the initial postoperative examination, regardless of the presence of amblyopia or the intermittency of the exodeviation.
Kushner and coworkers61 analyzed factors influencing response to strabismus surgery in the degrees of change of alignment per millimeter of surgery and found a positive correlation to the preoperative deviation in patients with exotropia and esotropia. The response was not significantly improved by including age, axial length, or preoperative refractive error. They stressed that although the response to strabismus surgery correlates significantly and inversely with axial length, that the preoperative deviation is a much stronger influence.
The surgeon who performs the recession-resection procedure must not anticipate more than a few prism diopters of immediate postoperative overcorrection. However, the surgeon who strives for “straight eyes” immediately after recession of the lateral rectus muscles consistently produces an undercorrection that requires secondary surgery for the residual exoangle. The surgeon who performs recession of the lateral rectus muscles must be prepared to accept transient postoperative esotropia for the first 10 days to 3 weeks if doing an adequate quantity of surgery. By contrast, Pratt-Johnson34 and associates statistically analyzed their data and found no significant difference in the immediate postoperative period, whether the eyes were esotropic, exotropic, or orthophoric. The possibility of persistent overcorrection with production of the monofixation syndrome, with or without amblyopia, or of an esotropia without fusion, must be considered.
In a series of 69 patients who were initially overcorrected, intentionally, after surgery for intermittent exotropia, Keech and Stewart62 found only eight patients (11.6%) with persistent overcorrection of 3PD or more, and only three (4.3%) with persistent diplopia. Smoot and colleagues63 reported restoration of stereopsis in eight of nine patients who required medial rectus recessions for constant secondary esotropia after bilateral lateral rectus muscle recessions for intermittent exotropia. Stereopsis measured 50 seconds or better on six patients, and 200 and 400 seconds in the other two patients who regained stereopsis. Unilateral or bilateral advancement of the medial rectus muscle to the original muscle insertion64 improved adduction deficiency in 71%, improved convergence insufficiency in 45%, and produced binocular vision in 50% when tested with Bagolini glasses. Secondary exotropia with limitation of adduction, either early, several weeks after surgery, or late, several months after surgery, require careful observation. Further surgery is more likely, as compared with the situation in patients without limitation of adduction.65
The presence of the monofixation syndrome after the surgical correction of intermittent exotropia may be explained by the presence of a preoperative monofixation syndrome.66 The intermittent nature of the exotropia produces the monofixation syndrome less frequently than does esotropia, in which the duration is more constant.67,68
In their outcome study of lateral rectus recession for intermittent exotropia in children, Ing and colleagues69 considered successful alignment to be an absence of any postoperative intermittent or constant tropia in any position of gaze. Their success rate was 62% (32 of 52) for the initial procedure, with a mean preoperative distance deviation of 25PD, a mean of 4 years and 8 months, and a mean follow-up of 4 years and 4 months. Age at initial surgery, initial deviation, initial refraction, and alignment 1 week postoperatively were not predictive of success. However, alignment at 6 months was highly correlated with successful alignment. Secondary surgery was required in 21% (11 of 52), for residual deviation, and an incidence of overcorrection of 17% (9 of 52) occurred, of which 8% (4 of 52) developed the monofixation syndrome. In the discussion of the Ing group's paper, Parks70 included those four patients with monofixation syndrome with excellent alignment as successful, with no deviation to 2PD of esodeviation at distance and 2 to 6 PD esodeviation at near. This increased the success rate to 69% (36 of 52) and reduced the overcorrections to 10% (5 of 52).
Undercorrections are more numerous than overcorrections,35 according to the quantity of surgery advised per prism diopter of deviation (see Table 1). According to Table 1, in approximately 5% of patients with recessions of the lateral rectus muscles as the initial surgery permanent overcorrection remains, compared with approximately 27% of patients who have undercorrection and who require secondary surgery. Patients with undercorrections of 15PD or more undergo reoperation with resections of the medial rectus muscles any time it becomes apparent that secondary surgery is needed, 6 weeks or later after initial surgery. If the initial surgery is a recession-resection procedure, then the secondary surgery is a recession-resection procedure on the opposite eye.
Associated motility problems are treated surgically at the time of exosurgery, except for overacting superior oblique muscles. A and V patterns are corrected by vertically offsetting the horizontal rectus muscles insertions, and overacting inferior oblique muscles are recessed. Overacting superior obliques are largely ignored in patients with intermittent exotropia because of previous bad experience in producing a postoperatively vertical deviation in primary position; this deviation causes conspicuous torticollis in some patients.
Sustained overcorrection of the exoangle deserves prolonged observation, antiaccommodation therapy, compensation of the esoangle with prism spectacles, and occlusion therapy for amblyopia.35,36,59,71–73 This therapy is initiated by the sixth postoperative week, unless it is apparent that straight eyes occur when raising or lowering the chin because of an associated A or V pattern. Miotic therapy rather than spectacles is usually used for this disorder because within a few days it is apparent whether or not antiaccommodation treatment improves the esoangle. If the miotic does not control the esotropia, prism spectacles are prescribed that also fully correct the hypermetropia, incorporating base-out prism power equal to that of the esoangle. If a concomitant high AC/A ratio is present, a miotic is used in conjunction with spectacles fitted with prisms. Prism power is reduced as the esoangle reduces; the Fresnel prism is ideal for this because of the ease in making the change. The patient is reexamined at monthly intervals, and if no improvement occurs within 6 months, surgical intervention is recommended. The surgeon is advised to follow the dictum of Cooper, approaching the case as though it were an unoperated esotropia, unless a duction restriction is apparent in the field of action of the previously operated muscle, requiring investigation and repair.74
EXOTROPIA
Management of exotropia includes treatment of amblyopia and surgery. Patients younger than 10 years of age may have their amblyopia improved before surgery, but most constantly exotropic patients have either alternate fixation or associated pathologic changes that explain the poor sight; therefore, only a minority require therapy for amblyopia.
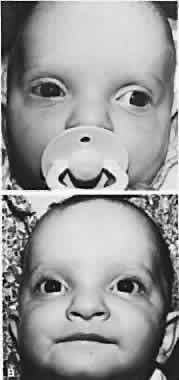
Patients with congenital constant exotropia should receive surgery as early as 6 months of age, and sufficient surgery should be performed to straighten the eyes, which usually are widely divergent. These patients are managed in a manner similar to those with congenital esotropia (Fig. 1). Infantile exotropia is rare,75 but high levels of binocular function can develop postoperatively in some patients. Oblique muscle overaction and dissociated vertical deviations are common, but nystagmus is rare, compared with congenital esotropia. Hunter and Ellis76 evaluated the prevalence of systemic and ocular disease associated with infantile exotropia. Records of 70 patients diagnosed with exotropia and 136 with esotropia before 1 year of age were evaluated. Some 67% with exotropia and 49% with esotropia had coexisting ocular or systemic abnormalities. Those with constant infantile exotropia were more likely to present with coexisting ocular and systemic disease than those with intermittent exotropia. Ocular diseases found in association with infantile exotropia included congenital cataracts, congenital nystagmus, Duane syndrome, types I and II, iridolenticular abnormalities, ocular motor apraxia, oculocutaneous albinism, optic atrophy, optic nerve hypoplasia, ptosis, and retinoschisis.
Children with an exotropic poor-seeing or blind eye that is cosmetically disfiguring should receive surgery by 4 years of age to prevent development of psychological problems resulting from the child's unusual appearance and the comments peers may make about the eyes.
Older patients with good vision in each eye receive surgery according to the same criteria discussed for patients with intermittent exotropia. Those with a poor-seeing or blind eye invariably receive recession-resection on that eye. If the exoangle of the poor eye exceeds 30PD, a surgeon is unable to limit the quantity of surgery per muscle to the quantity that does not interfere postoperatively with the normal horizontal ductions of the eye.
The surgeon must do excessive recession-resection to straighten the large unilateral exotropia, thus sacrificing normal postoperative abduction, or must be allowed by the patient to distribute the surgery between the two eyes; this means also operating on the better-seeing eye.77 The latter procedure is the best for motility status, but it may be disturbing to the patient. If the surgeon must confine the surgery to the poor-seeing eye, then the quantities listed in Table 2 are recommended.
TABLE 2. Quantity of Surgery Advised According to Exoangle in Patient with
Profound Amblyopia
| XT Angle | Recess LR | Resect MR |
| 40 | 8 mm | 6 mm |
| 50 | 9 mm | 7 mm |
| 60 | 10 mm | 8 mm |
| 70 | 10 mm | 9 mm |
| 80 | 10 mm | 10 mm |
LR, Lateral rectus; MR, medial rectus.
In addition to the muscle surgery required to correct the long-standing large angle of monocular exotropia, the tightly contracted lateral conjunctiva and Tenon's capsule must be recessed; otherwise an undercorrection may occur.
Raab described four-muscle surgery for large angle exotropia; this included recessing the lateral rectus 7 to 8 mm, resecting the medial rectus 6 to 7 mm, recessing the inferior oblique, and performing a tenectomy or tenotomy of the superior oblique.78 Additional temporal conjunctival recession was performed. Postoperative hypertropia may be a disadvantage and is avoided by infraplacement of both horizontal recti, ignoring an A or V pattern.
An important factor that alters the usual quantity of surgery per prism diopter of exoangle is the size of the eyeball, with microphthalmia requiring relatively less surgery.
In adults with long-standing horizontal strabismus and absence of fusion, a prospective, randomized clinical trial was reported by Carruthers and coworkers.79 Botulinum treatment was received by 17 and surgery with adjustable sutures by 13 patients. The net change in alignment was 50.3% with Botulinum, but 96% with surgery. A final deviation of 10PD or less at least 6 months after treatment was considered a successful outcome. Although the series was small, the success rate for Botulinum was only 29.4% compared with 76.9% with adjustable suture surgery.