However, in a retrospective study, Ing and co-workers5 evaluated 54 children with oculomotor nerve (CNIII) palsy, presenting over a period of 21 years, and found 38 isolated third CN lesions and 16 with additional CNs involved. Only 11 cases were congenital, and of the 43 that were acquired, 31 were traumatic, 7 related to infection, 2 ophthalmoplegic migraine, 2 neoplastic, and 1 vascular-hypertensive. In contrast with adults, this series did not include any third CN palsy secondary to aneurysm, diabetes, metastatic tumors, or pituitary lesions.
CONGENITAL
Congenital third CN palsy presents with varied degrees of extraocular involvement. Intraocular musculature is not usually affected in congenital third CN palsy, although pupil constriction may occur on attempted adduction in some cases of aberrant regeneration.6,7
The involved pupil may even be smaller than the uninvolved eye in congenital third CN misdirection syndrome.8 Although congenital third CN palsies are considered to be benign and isolated, Balkan and Hoyt9 reported other signs of focal neurologic damage, including pupillary involvement, oculomotor synkinesis, hemiplegia, seizures, and developmental delay.9 These congenital third CN palsies probably occur as a result of damage to both the peripheral nerve and the brain stem.9
Balkan and Hoyt found neurologic involvement or developmental delay in 7 of 10 patients studied. Hamed10 described neurologic involvement in 10 of 14 cases with congenital oculomotor palsy, and Tsaloumas and Willshaw11 reported 5 of 14 patients with congenital oculomotor palsy with significant neurologic abnormalities, including 2 patients using digital lid elevation to allow fixation with their affected eye.
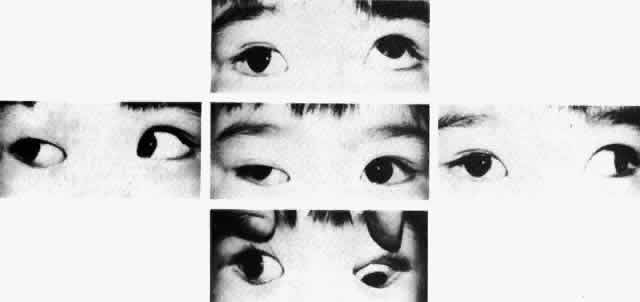
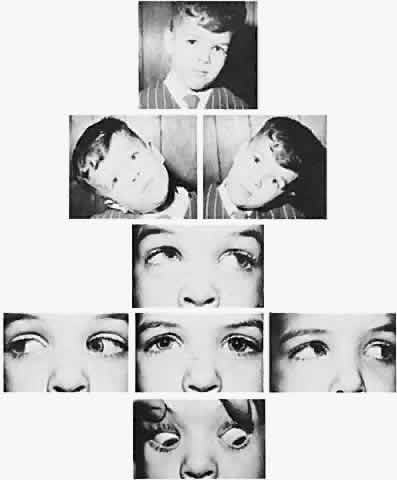
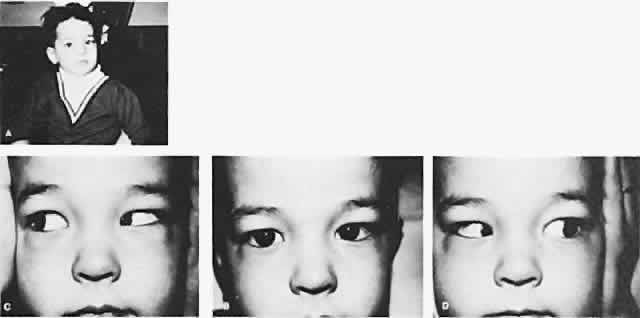
The degree of involvement of the levator muscle varies, but some function is usually retained. Therefore, ptosis is variable in this form of third CN palsy. The four extraocular muscles innervated by the third CN are also affected in various degrees. However, there is usually some trace at least of weakness of the medial, inferior, and superior rectus muscles, and of the inferior oblique muscle. The fourth CN is uninvolved and, consequently, the involved eye is exotropic and hypotropic (Fig. 1). Therefore, the clinician should always suspect congenital third CN palsy in an exotropic patient who has one low eye, intact pupillary and accommodation responses, and minimal ptosis of the involved eye with varied degrees of limitation of both elevation and depression in addition to diminished adduction. Many of these patients are able to develop single binocular vision and to maintain a compensatory malposition of the head that allows the alignment of the eyes to serve this purpose. When the eyes are moved into a position in which fusion is not possible, these patients experience diplopia if they have binocular vision with torticollis. Amblyopia of either eye may occur if the patient does not have binocular vision and does not maintain torticollis.
Schumacher-Feero and coworkers12 reported a series of 49 children with third CN palsy, involving 53 eyes, and observed during a follow-up for a mean of 5.5 years. Amblyopia developed in 27 eyes, and at the last follow-up visit, in 56% of affected eyes, the visual acuity ranged from 20/15 to 20/40. Binocular function was difficult to restore or to preserve but was significantly improved after surgery. Horizontal alignment was initially good in 6 of 49 patients, improving to 30 with good alignment at their last evaluation. The vertical alignment improved from 24 initially to 35 with good alignment at their last evaluation. Only 1 child achieved fusion at distance and near after a single recession/resection procedure at 8 months of age. A complete palsy required a mean of 2.3 operations to align the eyes, and a partial third CN palsy required a mean of 1.5 operations over the 5.5-year period. In general, the surgery was a horizontal recession/resection procedure for exotropia, with graded supraplacement of the horizontal rectus insertions for the hypotropia.
The cause of congenital third CN palsy is unknown, but it is presumed to be due to a developmental defect in either the nuclear or the motor fiber portion of the third CN complex that innervates the levator muscle and the extraocular muscles. It is not an extremely rare motility disorder. We have seen many patients having only unilateral involvement.
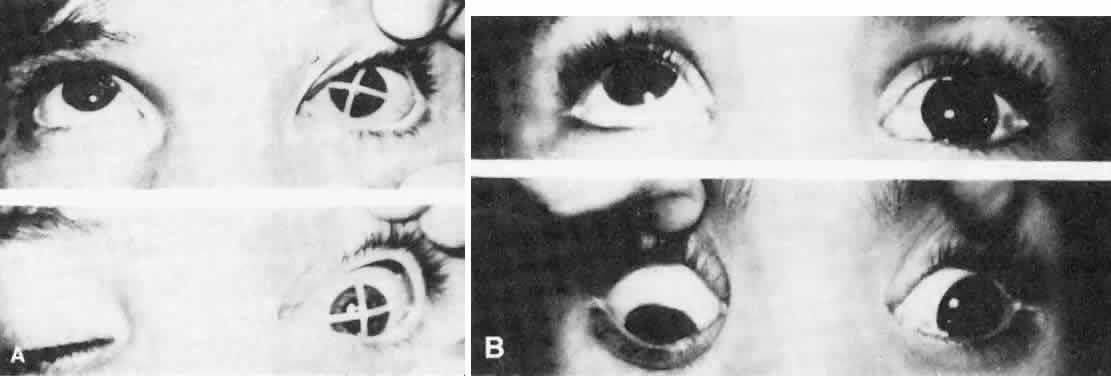
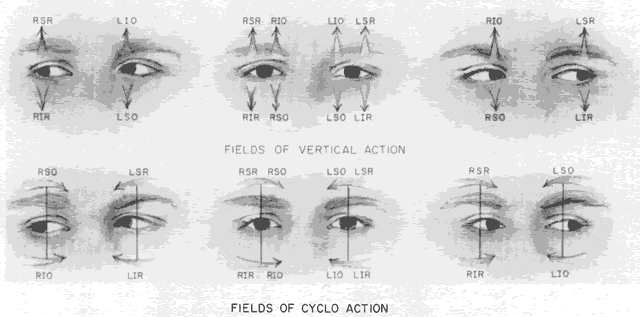
As in any third CN palsy, the absence of adduction of the involved eye makes it difficult to determine the intactness of the ipsilateral CN nerve. Clinically, the method used is to observe the crypts of the iris while the involved eye remains abducted and to ask the patient to look upward and downward. If the fourth CN is intact, the iris markings reveal a conspicuous intorsion as infraduction is attempted and extorsion as supraduction of the involved eye is attempted (Fig. 2). Dieterich and Brandt13 measured ocular torsion and subjective visual vertical tilts in acute and chronic oculomotor, trochlear, and abducens nerve palsies for each eye separately in the primary position with the head held upright. Unexpectedly, ocular torsion was abnormal in only 32% of third and fourth CN palsies involving oblique eye muscles, and normal in all abducens palsies. When measurable, pathologic ocular torsion was low, from 2 to 8 degrees, monocular, involving either the paretic or nonparetic eye. Subjective visual vertical tilts were abnormal in 67% of third and fourth CN palsies, mostly low in amplitude, between 1 and 6 degrees, and involving either the paretic or nonparetic eye, depending on the duration of the palsy. In contrast with acute unilateral brain-stem lesions with frequent binocular and conjugate tilts, third and fourth CN palsies cause only minor and unpredictable ocular torsion and subjective visual vertical tilts.
|
The forced duction test produces a negative result in third CN palsy; this rules out any adhesive phenomenon that limits motility of the eye. The degree of involvement of the third CN determines whether therapy is indicated. Involvement may be so minor and partial that no therapy is necessary, or it may affect only the elevators of an eye and is therefore known as double elevator palsy. Cadera and associates14 studied pathophysiology of double elevator palsy in two patients with magnetic resonance imaging (MRI) with volume scanning technique. They found the volume of the superior rectus muscle on the affected side to be less than half that of the normal eye, with other rectus muscles normal, suggesting either congenital hypoplasia or paresis of the affected superior rectus muscle. The inferior oblique muscles could not be evaluated by MRI. After a Knapp procedure in both patients, only minimal superior displacement of the medial and lateral rectus muscles was detectable posterior to the equator of the globe in both patients with MRI.
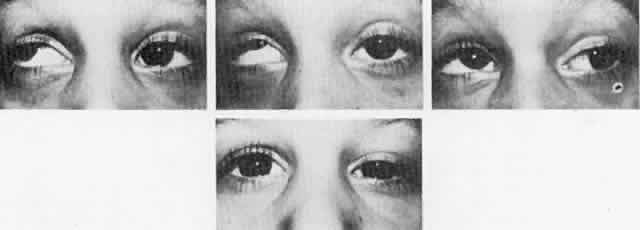
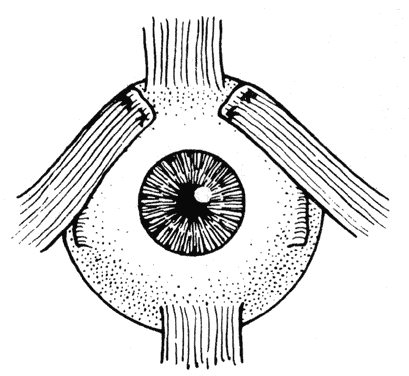
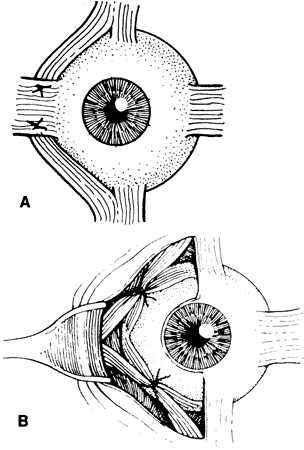
Double elevator palsy is usually rather complete, and it may also be associated with various degrees of ptosis (Fig. 3). The ptosis may be only pseudoptosis because of the hypotropia and because the lid position follows that of the eye. Fixating with the hypotropic eye causes the complete disappearance of the pseudoptosis; however, there may be a small degree of bona fide ptosis in addition to the pseudoptosis. The traction test result is normal in double elevator palsy. Treatment of double elevator palsy involves transposition of the insertions of the horizontal rectus muscles, placing the new insertions immediately adjacent to the insertion of the superior rectus muscle (Fig. 4). This does not produce normal elevation beyond the midline level, but it renders considerable improvement in, if not total elimination of, the hypotropia caused by this disorder.
After a full tendon transfer of the lateral and medial rectus muscle for double depressor or double elevator palsy, Knapp obtained an average correction of 38 diopters in the primary position and movement of 25° in the field of action of the paretic muscle group from the primary position.15 In the presence of a poor or absent Bell's phenomenon, an accentuated lower lid fold of the hypotropic eye in attempted elevation, and a positive traction test result, Scott and Jackson recommend inferior rectus recession only as the initial procedure.16 With a negative forced traction test result, a full Knapp procedure is advised.
Complete congenital third CN involvement requires surgery for exotropia, hypotropia, and ptosis. Hypotropia is resolved by disinserting the tendon of the superior oblique muscle from the globe, which is tight and contracted. Maximal recession of the lateral rectus and resection of the medial rectus may be sufficient to reposition the involved eye in the horizontal plane satisfactorily. However, if this is inadequate, removing the superior oblique tendon from the trochlea, severing the reflected tendon of the superior oblique muscle from the muscular portion, and attaching the superior oblique muscle to the sclera at the insertion of the medial rectus muscle offer excellent correction of the horizontal defect created by the third CN palsy in the primary position.
This does not create normal adduction of the involved eye but is an effective technique for centering the eye.17 However, following transposition of the superior oblique muscle, when the patient depresses the involved eye, it adducts. Saunders and Rogers, who attempted correction of third CN palsy by superior oblique anterior transposition and advancement without trochleotomy, reported unsatisfactory results because of inadequate horizontal alignment, postoperative hyperdeviations, or paradoxical ocular movements.18 Superior oblique tendon transposition with trochleotomy causes the adherence syndrome, owing to violation of Tenon's capsule, which is unavoidable in removing the superior oblique tendon from the trochlea. Therefore, this procedure is no longer recommended. Scott and colleagues19 and Gottlob and associates20 described anterior transposition of the nasal portion of the superior oblique tendon, 2 to 3 mm anterior to the nasal border of the superior rectus muscle, without trochleotomy. In addition, Gottlob and associates20 performed large recessions of the ipsilateral lateral rectus, and in some patients, a recess-resect procedure on the horizontal rectus muscles of the contralateral eye. Orthophoria was achieved in 4 patients, a 10 prism diopter (PD) residual exotropia in 1 patient, and 2 patients required reoperations because of aberrant regeneration of the oculomotor nerve. In a much larger series, Maruo and coworkers21 compiled 280 cases of exotropia secondary to oculomotor palsy, between 1971 and 1993. There were 130 congenital and 150 acquired cases of oculomotor palsy. Surgery was performed 234 times in 138 patients with paralytic exotropia, with transposition of the superior oblique tendon and resection of the medial rectus muscle, with or without resection of the ipsilateral lateral rectus muscle. With a 4-year follow-up in 35 cases, the authors found similar results when transposing the superior oblique tendon in patients with complete palsy, and in resection of the medial rectus muscle in patients with incomplete palsy. There was no benefit adding a resection of the medial rectus when the superior oblique transposition was performed. However, recession of the lateral rectus muscle greatly improved the effectiveness of either the superior oblique transposition or the medial rectus resection. Mudgil and Repka22 reviewed retrospectively the ophthalmologic outcome of third CN palsy or paresis in 41 children younger than 8 years of age. Etiologies included congenital in 39%, traumatic in 37%, and neoplastic in 17%. Initial visual acuities were reduced in 71%, and the long-term outcome in the 20 who could be observed during follow-up, for a mean of 3.6 years, vision was reduced in 35% because of amblyopia, and in 25% because of nonamblyopic factors. In the congenital third CN palsy group, all patients improved to normal visual acuity. Despite improved alignment after surgery in 40% (8 of 20) of children with long-term follow-up, none obtained measurable stereopsis. Aberrant reinnervation occurred in 45% (9 of 20). Only 3 patients fully recovered and regained measurable stereopsis, with the etiologies of congenital, neoplastic, and traumatic third CN palsies.
Because of disruption in sensory fusion mechanisms, Elston has recommended a one-stage procedure of maximal lateral rectus recession, medial rectus resection, and simultaneous transposition of insertions to that of the superior rectus.23 The simplest procedure providing predictable cosmetic improvement has been recommended. Ptosis, however, was not addressed. A frontalis suspension of the ptotic lid is the indicated procedure for the associated ptosis. A synthetic material (e.g., 4-0 Supramid) is ideal because if the cornea cannot tolerate the relatively dry state after the lid is elevated, these synthetic sutures can easily be removed and the cornea not harmed permanently. The surgeon should be aware of the absence of Bell's phenomenon in these patients and be alert to postoperative corneal problems associated with this deficiency.
ACQUIRED
Acquired third CN palsy may be partial or complete and may involve only the extraocular muscles or both intraocular and extraocular muscles. The pupil is usually spared in third CN palsy associated with diabetes.
Acquired third CN palsy usually occurs rather precipitously with maximal involvement. Within days to weeks, there may be an indication of restoration of third CN function manifest by only partial involvement. Recovery is usually complete by 6 months following onset, and, consequently, no judgment should be rendered regarding the necessity of treatment until after the 6-month interval. Partial or complete recovery can be expected, depending on the etiology of the third CN palsy, in 48% of the patients.3 Many times such palsy results from relatively serious intracranial involvement, and this may determine whether therapy is indicated. Mark24 recommends MRI evaluation of patients with third CN palsy to include proton density and T2-weighted images through the brain in axial section, to study the brain stem for nuclear lesions, along with thin section T1-weighted images in the coronal and axial planes to evaluate cisternal, cavernous, and orbital segments of the third nerve. Gadolinium diethylenepentaminetetraacetic acid has also been found helpful in evaluation of third CN palsy. In posttraumatic third CN palsies, use of gradient echo images to detect hemorrhage is helpful. Ischemia of the oculomotor nerve causes most cases of nontraumatic oculomotor nerve palsy.25 MRI and lumbar puncture are helpful in diagnosing cases caused by inflammatory or neoplastic meningitis. A cerebral aneurysm, which can be fatal, can be diagnosed by cerebral angiography, but this test has a 1% to 2% morbidity and mortality rate. Magnetic resonance angiography is a variant of MRI that highlights blood vessels; however it is only 95% accurate in detection of aneurysms. Trobe25 therefore concludes that because pupil involvement occurs in 96% with aneurysms, and if anisocoria exceeds 2.0 mm, that catheter angiography is justified.
The causes can be classified as follows:
VII. M.
- Brain-stem lesion
- Benedikt's syndrome manifest by homolateral third CN paralysis and
contralateral intention tremor
- Weber's syndrome manifest by homolateral third CN paralysis and contralateral
hemiplegia
- Benedikt's syndrome manifest by homolateral third CN paralysis and
contralateral intention tremor
- Inflammatory conditions
- Meningitis
- Encephalitis
- Polyneuritis from toxins such as alcohol, lead, arsenic, and carbon monoxide, and
from diabetes
- Herpes zoster infection
- Echovirus infection26
- Meningitis
- Vascular lesions
- Tumors1–3,32
- Glioblastoma multiforme33
- Glioblastoma multiforme33
- Demyelinating diseases
- Trauma
- Miscellaneous
- Anterior communicating artery aneurysm34,36
- Bilateral chronic subdural hematomas34,37
- Congenital toxoplasmosis38
- “Crack” cocaine39
- Diagnostic angiography34,40
- Eosinophilic granuloma of the optic nerve34,41
- Frontal sinus mucocele42
- Infectious mononucleosis43
- Leukemia34,44
- Measles immunization45
- Myasthenia gravis46,47
- Ophthalmoplegic migraine5,48–50
- Polyarteritis nodosa
- Porphyria
- Sarcoidosis
- Schwannoma34,51
- Temporal arteritis52
- Viagra therapy (sildenafil citrate)53
- Anterior communicating artery aneurysm34,36
Partial third CN palsy in the form of isolated inferior rectus paresis may be the presenting sign of myasthenia gravis, with sudden onset of diplopia.47 The oculomotor nerve aberrant regeneration or misdirection syndrome is believed to result from extensive and haphazard growth that characterizes the regeneration of injured nerve fibers.8,23 The third CN misdirection syndrome includes the following:8
- Retraction of the globe on attempted vertical gaze
- Adduction of the globe on attempted vertical gaze
- Upper lid retraction on attempted downgaze (pseudo-Graefe's sign)
- Narrowing of the fissure on abduction, and widening of the fissure on attempted
adduction (horizontal gaze lid dyskinesis)
- Pseudo-Argyll Robertson pupil
- Relative monocular vertical optokinetic responses
Treatment involves relief of the patient's diplopia, which usually is not a problem in complete third CN paralysis because of the associated ptosis covering the pupil. However, in partial involvement, the lid may sufficiently clear the pupillary space so that diplopia is a problem. Occlusion therapy is the best solution for the patient's diplopia. The patient usually wishes to have the involved eye occluded rather than the uninvolved eye. Surgery is indicated for associated strabismus and ptosis if the patient's general condition permits it and if a significant residual paralysis is present 6 months after onset of third CN palsy. The surgery described for congenital third CN palsy is also applicable for acquired third CN palsy. Kushner54 described surgical treatment in five patients with the rare finding of paralysis of the inferior division of the third CN. Clinical findings included a large exotropia and hypertropia of the affected eye, intorsion, and internal ophthalmoplegia. As described by Knapp,55 Kushner performed a superior oblique tenotomy along with transposing the superior rectus toward the insertion of the superior border of the medial rectus, following the spiral of Tillaux and transposing the lateral rectus toward the lateral border of the inferior rectus, following the spiral of Tillaux. In follow-up lasting between 3 and 10 years, all patients maintained satisfactory eye alignment and were free from diplopia in the primary position.