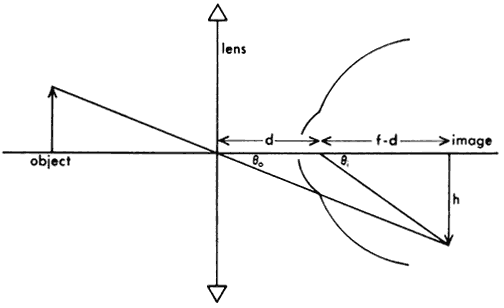
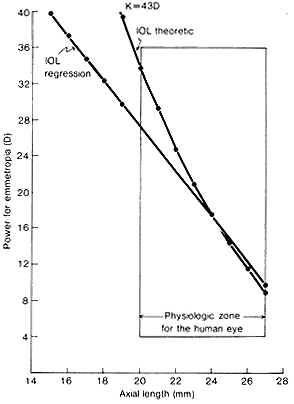
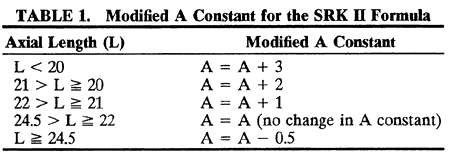
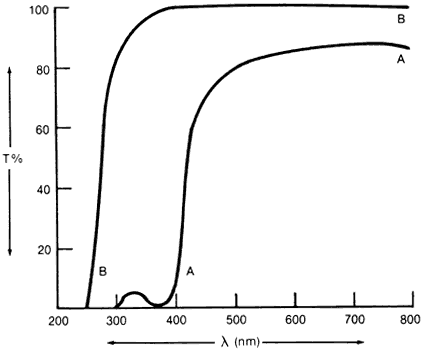
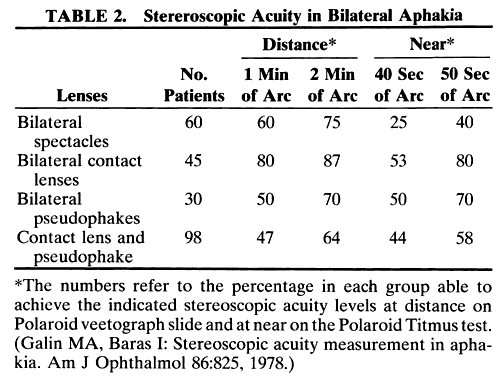
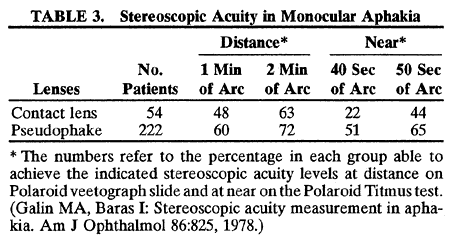
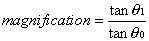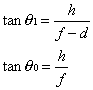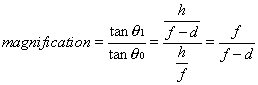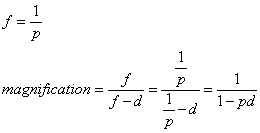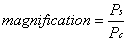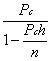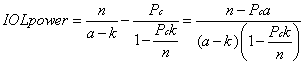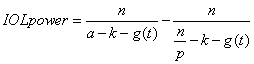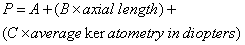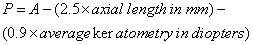1. Casanova de Seingalt G: Memoirs (translated by Machen A). Vol 8. New York: Limited
Editions Club, 1940:47 2. Ridley H: Intraocular acrylic lenses. Trans Ophthalmol Soc UK 71:617, 1951 3. Ridley H: Intra-ocular acrylic lenses after cataract extraction. Lancet 1:118, 1952 4. Binkhorst RD: The optical design of intraocular lens implants. Ophthalmic Surg 6:17, 1975 5. Ogle KN, Burian HM, Bannon RE: On the correction of unilateral aphakia with contact lenses. Arch Ophthalmol 59:639, 1958 6. Schechter RJ: Image magnification, contact lenses, and visual acuity. Ann Ophthalmol 10:1665, 1978 7. Ogle KN: Optics, p 192. 2nd ed. Springfield, IL: Charles C Thomas, 1968 8. Ridley H: Intraocular acrylic lenses: Past, present, and future. Trans Ophthalmol Soc UK 84:5, 1964 9. Etienne CE: A micro-computer program package for the computation of intraocular lens
powers. Ophthal Surg 15:386, 1984 10. Paviin CJ: Use of electronic spread-sheet programs for intraocular lens power calculation. Ophthalmic Surg 15:58, 1984 11. Thompson JT, Maumenee AK, Baker CC: A new posterior chamber intraocular lens formula for axial myopes. Ophthalmology 91:484, 1984 12. Murphy CG, Murphy GE: A BASIC program for deriving linear regression formulas for intraocular
lens power prediction. J Cataract Refract Surg 12:188, 1986 13. Lugo M: A simple BASIC computer program to individualize the SRK formula. Arch Ophthalmol 104:687, 1986 14. Sharvelle DJ: A BASIC language computer program for intraocular lens power calculations. Am Intraocular Implant Soc J 11:400, 1985 15. McEwan JR, Cinotti DJ, Maltzman BA: Estimates of primary implant power using an intraocular lens table. J Cataract Refract Surg 12:401, 1986 16. Want G, Pomerantzeff O, Miao T: A slide rule for calculating the power of an intraocular lens. Am Intraocular Implant Soc J 9:335, 1983 17. McDonald JE II: A menu-driven Lotus 1-2-3 template for intraocular lens calculation and
automatic generation of an SRK formula. Ann Intraocular Implant Soc J 11:75, 1985 18. Binkhorst CD: Intraocular lens power. Trans Am Acad Ophthalmol Otolaryngol 81:70, 1976 19. Ogachi Y. van Balen AThM: Determination of the expected power of the implant lens by ultrasound. Ophthalmologica 171:281, 1975 20. Fyodorov SN, Galin MA, Linksz A: Calculation of the optical power of intraocular lenses. Invest Ophthalmol Vis Sci 14:625, 1975 21. Binkhorst RD: Pitfalls in the determination of intraocular lens power without ultrasound. Ophthalmic Surg 7:69, 1976 22. van der Heijde GL: A nomogram for calculating the power of the pre-pupillary lens in the aphakic
eye. Bibl Ophthalmol 83:273, 1975 23. van der Heijde GL: The optical correction of unilateral aphakia. Trans Am Acad Ophthalmol Otolaryngol 81:80, 1976 24. Olsen T: Theoretical vs empirical prediction of aphakic refraction. Arch Ophthalmol 105:1042, 1987 25. Olsen T: Theoretical, computer-assisted prediction versus SRK prediction of postoperative
refraction after intraocular lens implantation. J Cataract Refract Surg 13:146, 1987 26. Hillman JS: Intraocular lens power calculation for emmetropia: A clinical study. Br J Ophthalmol 66:53, 1982 27. Leonard PAM: Ultrasonography and lens implantation. Ophthalmologica 171:726, 1975 28. Weinstein GW, Baum G. Binkhorst RD et al:A comparison of ultrasonographic and optical methods for determining the
axial length of the aphakic eye. Am J Ophthalmol 62:1194, 1966 29. Hoffer KJ: Accuracy of ultrasound intraocular lens calculation. Arch Ophthalmol 99:1819, 1981 30. Hoffer KJ: Preoperative cataract evaluation: Intraocular lens power calculation. Ophthalmol Clin 22:37, 1982 31. Hoffer KJ: Intraocular lens calculation: The problem of the short eye. Ophthalmol Surg 12:269, 1981 33. Fritz KJ: Letter: Intraocular lens power formulas. Am J Ophthalmol 91:414, 1981 34. Retzlaff J: A new intraocular lens calculation formula. Am Intraocular Implant Soc J 6:148, 1980 35. Sanders DR, Kraff MC: Improvement of intraocular lens power calculation using empirical data. Am Intraocular Implant Soc J 6:263, 1980 36. Retzlaff J: Posterior chamber implant power calculation: Regression formulas. Am Intraocular Implant Soc J 6:268, 1980 37. Freudiger H, Artaria L, Niesel P: Influence of intraocular lenses on ultrasound axial length measurement: In
vitro and in vivo studies. Am Intraocular Implant Soc J 10:29, 1984 38. Lindstrom RL: Accuracy of lens implant power determination using A-scan. Contact Lens 5:61, 1979 39. Hillman JS: Intraocular lens power calculation: The selection of formula. Trans Ophthalmol Soc UK 104:693, 1985 40. Armstrong TA, Lichtenstein SB: Intraocular lenses in myopes. Ophthalmic Surg 15:653, 1984 41. Sanders D, Retzlaff J. Kraff M et al:Comparison of the accuracy of the Binkhorst, Colenbrander, and SRK implant
power prediction formulas. Am Intraocular Implant Soc J 7:337, 1981 42. Leaming DV: Practice styles and preferences of ASCRS members: 1999 Survey. J Cataract Refract Surg 26:913, 2000 43. Hoffer KJ: Biometry of 7,500 cataractous eyes. Am J Ophthalmol 90:360, 1980 44. Fontana ST, Brubaker RF: Volume and depth of the anterior chamber in the normal aging human eye. Arch Ophthalmol 98:1803, 1980 45. Holladay JT, Musgrove KH, Prager TC et al: A three-part system for refining intraocular lens power calculations.J Cataract Refract Surg 14:17, 1988 46. Sanders DR, Retzlaff J, Kraff MC: Comparison of the SRK II formula and other second generation formulas. J Cataract Refract Surg 14:136, 1988 47. Olsen T, Thim K, Corydon L: Theoretical versus SRK I and SRK II calculation of intraocular lens power. J Cataract Refract Surg 16:217, 1990 48. McEwan JR, Massengill RK, Friedel SD: Effect of keratometer and axial length measurement errors on primary implant
power calculations. J Cataract Refract Surg 16:61, 1990 49. Retzlaff J, Sanders D, Kraff M: A Manual of Implant Power Calculation. Copyright
Retzlaff J, Sanders D, Kraff M. Distributed by Cilco Inc, 1982:17a–17b 50. Sanders DR, Retzlaff J, Kraff MC: Comparison of empirically derived and theoretical aphakic refraction formulas. Arch Ophthalmol 101:965, 1983 51. Holladay JT, Prager TC, Chandler TY et al: A three-part system for refining intraocular lens power calculations.J Cataract Refract Surg 14:17, 1988 52. Hoffer KJ: Lens power calculation for multifocal IOLs. In Maxwell WA, Nordan
LT (eds): Current Concepts of Multifocal Intraocular Lenses. Thorofare, NJ: Slack, 1991:193–208 53. Hoffer KJ: The Hoffer Q formula: A comparison of theoretic and regression formulas. J Cataract Refract Surg 19:700, 1993 54. Retzlaff JA, Sanders DR, Kraff MC: Development of the SRK/T intraocular
lens implant power calculation formula. J Cataract Refract Surg 16:333, 1990 [Errata: J
Cataract Refract Surg 16:528, 1990; J Cataract
Refract Surg 19:442, 1993] 55. Hoffer KJ: Clinical Results using the Holladay 2 intraocular lens power formula. J Cataract Refract Surg 26:1233, 2000 56. Inatomi M, Ishii K, Koide R et al: Intraocular lens power calculation for microphthalmos. J Cataract Refract Surg 23:1208, 1997 57. Andreo LK, Wilson ME, Saunders RA: Predictive value of regression and theoretical IOL formulas in pediatric
intraocular lens implantation. J Pediatr Ophthalmol Strabismus 34:240,1997 58. Fenzl RE, Gills JP, Cherchio M: Refractive and visual outcome of hyperopic cataract cases operated on before
and after implementation of the Holladay II formula. Ophthalmology 105:1759, 1998 59. MacKool RJ: The cataract extraction-refraction-implantation technique for
IOL power calculation in difficult cases. J Cataract Refract Surg 24:434, 1998 (Letter) 60. Binkhorst RD: Biometric A scan ultrasonography and intraocular lens power
calculation. In Emery JM (ed): Current Concepts in Cataract Surgery. St
Louis: CV Mosby, 1978:175–182 61. Olsen T: Calculating axial length in the aphakic and pseudophakic eye. J Cataract Refract Surg 14:413, 1988 62. Sanders DR, Kraff MC: A comparison of the Digital Biometric Ruler-300 and Echo-oculometer-3000: A
report of two hundred cases.Am Intraocular Implant Soc J 8:365, 1982 63. Richards SC, Olson RJ, Richards WL: Factors associated with poor predictability by intraocular lens calculation
formulas. Arch Ophthalmol 103:515, 1985 64. Naeser K, Naeser A, Boberg-Ans J et al: Axial length following implantation of posterior chamber lenses. J Cataract Refract Surg 15:673, 1989 65. Coleman DJ, Lizzi FL, Jack RL: Ultrasonography of the Eye and Orbit. Philadelphia: Lea & Febiger, 1977:91–129 66. Shammas HJF: Axial length measurement and its relation to intraocular lens power calculations. Am Intraocular Implant Soc J 8:346, 1982 67. Tabandeh H, Wilkins M, Thompson G et al: Hardness and ultrasonic characteristics of the human crystalline lens.J Cataract Refract Surg 26:838, 2000 68. Hoffer KJ: Ultrasound velocities for axial eye length measurement. J Cataract Refract Surg 20:554, 1994 69. Hoffer KJ: Axial dimension of the human cataractous lens. Arch Ophthalmol 111:914, 1993 70. Holladay JT: Standardizing constants for ultrasonic biometry, keratometry, and intraocular
lens power calculations. J Cataract Refract Surg 23:1356, 1997 71. Milauskas AT: Pseudo axial length increase after silicone lens implantation as determined
by ultrasonic scans. J Cataract Refract Surg 14:400, 1988 72. McCartney DL, Miller KM, Stark WJ et al: Intraocular lens style and refraction in eyes treated with silicone oil. Arch Ophthalmol 105:1385, 1987 73. Murray DC, Potamitis T, Good P et al: Biometry of the Silicone filled eye. Eye 13:319, 1999 74. Margin RG, Safir A: Asteroid hyalosis affecting the choice of intraocular lens implant. J Cataract Refract Surg 13:62, 1987 75. Erkin EF, Tarhan S, Ozturk F: Axial Length measurement and asteroid hyalosis. J Cataract Refract Surg 25:1400, 1999 76. Oguchi Y, van Balen ATM: Ultrasonic study of the refraction of patients with pseudophakos. Ultrasound Med Biol 3:267, 1974 77. Shelenz J, Kammann J: Comparison of contact and immersion techniques for axial length measurement
and implant power calculation. J Cataract Refract Surg 15:425, 1989 78. Giers U, Epple C: Comparison of A-scan device accuracy. J Cataract Refract Surg 16:235, 1990 79. Murphy GE, Murphy CG: Comparison of efficacy of longest-average, and shortest axial length measurements
with a solid-tip ultrasound probe in predicting intraocular
lens power. J Cataract Refract Surg 19:644, 1993 80. Berges O, Puech M, Assouline M et al: B-mode-guided vector-A-mode versus A-mode biometry to determine axial length
and intraocular lens power. J Cataract Refract Surg 24:529, 1998 81. Zaldivar R, Shultz MC, Davidorf JM et al: Intraocular lens power calculations in patients with extreme myopia. J Comput Assist Tomogr 26:668, 2000 82. Insler MS: Liability for intraocular lens calculations. Am J Ophthalmol 110:578, 1990 (Letter) 83. Drexler W, Findl O, Menapace R et al: Partial coherence interferometry: A novel approach to biometry in cataract
surgery. Am J Ophthalmol 126:524, 1998 84. Cashwell FL, Martin CA: Axial length decrease accompanying successful glaucoma filtration surgery. Ophthalmology 106:2307, 1999 85. Malukiewicz-Wisniewska G, Stafiej J: Changes in axial length after retinal detachment surgery. Eur J Ophthalmol 9:115, 1999 86. Cleasby GW, Dadson AA: The effects of hard contact lenses on intraocular lens power calculations. Am Intraocular Implant Soc J 11:603, 1985 87. Floyd G: Changes in the corneal curvature following cataract extraction. Am J Ophthalmol 34:1525, 1951 88. Shammas HJF: The fudged formula for intraocular lens power calculations. Am Intraocular Implant Soc J 8:350, 1982 89. Husain SE, Kohnen T, Maturi R et al: Computerized videokeratography and keratometry in determining intraocular
lens calculations. J Cataract Refract Surg 22:362, 1996 90. Katz HR, Forster RK: Intraocular lens calculation in combined penetrating keratoplasty, cataract
extraction and intraocular lens implantation. Ophthalmology 92:1203, 1985 91. Crawford GJ, Stulting RD, Waring GO et al: The triple procedure: Analysis of outcome, refraction, and intraocular
lens power calculation. Ophthalmology 93:817, 1986 92. Abdel-Hakin AS, Khalil A: Intraocular lens power calculations in the triple procedure. Br J Ophthalmol 73:709, 1989 93. Mattax JB, McCulley JP: The effect of standardized keratoplasty technique on IOL power calculation
for the triple procedure. Acta Ophthalmol 67(Suppl):24, 1989 94. Musch DC, Meyer RF: Prospective evaluation of a regression-determined formula for use in triple
procedure surgery. Ophthalmology 95:79, 1988 95. Koch DD, Liu IF, Hyde LL et al: Refractive complications of cataract surgery after radial keratotomy. Am J Ophthalmol 108:676, 1989 96. Celikkol L, Pavolpoulos G, Weinstein B et al: Calculation of intraocular lens power after radial keratotomy with computerized
videokeratography. Am J Ophthalmol 120:739, 1995 97. Kalski RS, Danjoux JP, Fraenkel GE et al: Intraocular lens power calculation for cataract surgery after photorefractive
keratectomy for high myopia. J Refract Surg 13:362, 1997 98. Gimbel HV, Sun R, Furlong MT et al: Accuracy and predictability of intraocular lens power calculation after
photorefractive keratectomy. J Cataract Refract Surg 26:1147, 2000 99. Hoffer KJ: Intraocular lens power calculation for eyes after refractive keratotomy. J Refract Surg 11:490, 1995 100. Zeh WG, Kock DD: Comparison of contact lens overrefraction and standard keratometry for
measuring corneal curvature in eyes with lenticular opacity. J Cataract Refract Surg 25:898, 1999 101. Seitz B, Langenbucher A, Nguyen NX et al: Underestimation of intraocular lens power for cataract surgery after myopic
photorefractive keratectomy. Ophthalmology 106:693, 1999 102. Speicher L, Gottinger W: Intraocular lens power calculation after decentered photorefractive keratectomy. J. Cataract Refract Surg 25:140, 1999 103. Panel discussion: Determination of intraocular lens power. In Emery JM, Jacobson
AC (eds): Current Concepts in Cataract Surgery: Selected Proceedings
of the Sixth Biennial Cataract Surgical Congress. St Louis: CV
Mosby, 1980:104–109 104. Shammas HJF: Postoperative anterior chamber depth for anterior chamber lenses. Am Intraocular Implant Soc J 6:153, 1980 105. Hoffer KJ: Biometry of the posterior capsule: A new formula for anterior
chamber depth of posterior chamber lenses. In Emery JM, Jacobson AC (eds): Current
Con-cepts in Cataract Surgery: Selected Proceedings of
the Eighth Biennial Cataract Surgical Congress. Norwalk, CT: Appleton-Century-Crofts, 1984:56 106. Naeser K, Boberg-Ans J, Bargum R: Biometry of the posterior lens capsule: A new method to predict pseudophakic
anterior chamber depth. J Cataract Refract Surg 16:202, 1990 107. Olsen T: Prediction of intraocular lens position after cataract extraction. J Cataract Refract Surg 12:376, 1986 108. Binkhorst RD, Weinstein GW, Troutman RC: A weightless iseikonic intraocular lens. Am J Ophthalmol 58:73, 1964 109. Cekic O, Batman C: The relationship between capsulor-rhexis size and anterior chamber depth
relation. Ophthal Surg Lasers 30:185, 1999 110. Olsen T, Gimbel H: Phacoemulsification, capsulorrhexis, and intraocular lens power prediction
accuracy. J Cataract Refract Surg 19:695, 1993 111. Hayashi K, Hayashi H, Nakao R et al: Intraocular lens tilt and decentration, anterior chamber depth, and refractive
error after transscleral suture fixation surgery. Ophthalmology 106:878, 1999 112. Findl O, Drexler W, Menapace R et al: Changes in intra-ocular lens position after neodymium:YAG capsulotomy. J Cataract Refract Surg 25:659, 1999 113. Hull CC, Liu CSC, Sciscio A: Image quality in polypseudophakia for extremely short eyes. Br J Ophthalmol 83:656, 1999 114. Gayton JL, Sanders VN: Implanting two posterior chamber intraocular lenses in a case of microphthalmos. J Cataract Refract Surg 19:776, 1993 115. Holladay JT, Gills JP, Leidlein J et al: Achieving emmetropia in extremely short eyes with two piggyback posterior
chamber intraocular lenses. Ophthalmology 103:1118, 1996 116. Gills JP: Piggyback minus-power lens implantation in keratoconus. J Cataract Refract Surg 24:566, 1998 117. Gayton JL, Sanders V, Van Der Kar M et al: Piggybacking intraocular implants to correct pseuodphakic refractive error. Ophthalmology 106:56, 1999 118. Findl O, Menapace R, Rainer G et al: Contact zone of piggyback acrylic intraocular lenses. J Cataract Refract Surg 25:860, 1999 119. Findl O, Menapace R: Piggyback intraocular lenses. J Cataract Refract Surg 26:308, 2000 (Letter) 120. Shugar JK, Schwartz T: Interpseudophakos Elschnig pearls associated with late hyperopic shift: A
complication of piggyback posterior chamber intraocular lens implantation.J Cataract Refract Surg 25:863, 1999. 121. Shimizu K, Misawa A, Suzuki Y: Toric intraocular lenses: Correcting astigmatism while controlling axis
shift. J Cataract Refract Surg 20:523, 1994 122. Ruhswurm I, Scholz U, Zehermayer M et al: Astigmatism correction with a foldable toric intraocular lens in cataract
patients. J Cataract Refract Surg 26:1022, 2000 123. Xiao-Yi S, Vicary D, Montgomery P et al: toric intraocular lenses for correcting astigmatism in 130 eyes. Ophthalmology 107:1776, 2000 124. Novis C: Astigmatism and the toric intraocular lines and other vertex distance effects. Surv Ophthalmol 42:268, 1997 125. Alio JL, de la Hoz F, Ruiz-Moreno JM et al: Cataract surgery in highly myopic eyes corrected by phakic anterior chamber
angle-supported lenses. J Cataract Refract Surg 26:1303, 2000 126. Perez-Santonja JJ, Alio JL, Jimenez-Alfaro I: Surgical correction of severe myopia with an angle-supported phakic intraocular
lens. J Cataract Refract Surg 26:1288, 2000 127. Budo C, Hessloehl JC, Izak M: Multicenter study of the artisan phakic intraocular lens. J Cataract Refract Surg 26:1163, 2000 128. Fechner PU, Haigis W, Wichmann W: Posterior chamber myopia lenses in phakic eyes. J Cataract Refract Surg 22:178, 1996 129. Lesueur LC, Arne JL: Phakic posterior chamber lens implantation in children with high myopia. J Cataract Refract Surg 25:1571, 1999 130. Holladay JT: Refractive power calculations for intraocular lenses in the phakic eye. Am J Ophthalmol 116:63, 1993 131. Ben-Ezra D, Cohen E, Karshai I: Phakic posterior chamber intraocular lens for the correction of anisometropia
and treatment of amblyopia. Am J Ophthalmol. 130:292, 2000 132. Lesiewska-Junk H, Kaluzny J: Intraocular lens movement and accommodation in eyes of young patients. J Cataract Refract Surg 26:562, 2000 133. Nakazawa M, Ohtsuki K: Apparent accommodation in pseudophakic eyes after implantation of posterior
chamber intraocular lenses: optical analysis. Invest Ophthalmol Vis Sci 25:1458, 1984 134. Legeais JM, Werner L, Werner L et al: Pseudoaccommodation: BioComFold versus a foldable silicone intraocular
lens. J Cataract Refract Surg 25:262, 1999 135. Nishi O, Nishi K: Accommodation amplitude after lens refilling with injectable silicone by
sealing the capsule with a plug in primates. Arch Ophthalmol 116:1358, 1998 136. Norrby NES, Grossman LW, Geraghty EP et al: Determining the imaging quality of intraocular lenses. J Cataract Refract Surg 24:703, 1998 137. Gills JP: Letter to the editor. Ann Intraocular ImplantSoc J 4:163, 1978 138. Lloyd T, Montgomery D, Gills JP: Deviation from labeled dioptic power for 400 lenses. Am Intraocular Soc J 5:229, 1979 139. Miller D. Manning, Miller R et al: Intraocular lens power check. Am J Ophthalmol 91:462, 1981 140. Holladay JT, Van Gent S, Ting AC et al: Silicone intraocular lens power
vs. temperature. Am J Ophthalmol 107:428, 1989 (Letter) 141. Holladay JT, Prager TC, Long SA et al: Determining intra-ocular lens power within the eye. Am Intraocular Implant Soc J 11:353, 1985 142. Olsen R: Measuring the power of an in situ intraocular lens with the keratometer. J Cataract Refract Surg 14:64, 1988 143. Binkhorst CD, Colenbrander MC, Loones LH: Determination of the power of a convex-piano intraocular lens in situ from
the dioptric keratometer readings of its front surface: Extension
table for the Javal-Schiotz ophthalmometer. Br J Ophthalmol 71:473, 1987 144. McReynolds WU, Snider NL: The quick, simple measurement of intraocular lens power and lens resolution
at surgery. Am Intraocular Implant Soc J 4:15, 1978 145. Olson RJ, Drandall AS, Welch RC: Intraocular lens quality control. Am Intraocular Implant Soc J 8:361, 1982 146. Kohnen S: Postoperative refractive error resulting from incorrectly labeled intraocular
lens power. J Cataract Refract Surg 26:777, 2000 147. Olson RJ, Kolodner H, Kaufman HE: The optical quality of currently manufactured intraocular. Am J Ophthalmol 88:548, 1979 148. Olson RJ: Intraocular lens optical quality: Update 1979. Am Intraocular Implant Soc J 6:16, 1980 149. Sivak JG, Kreuzer RO, Hildebrand T: Intraocular lenses, tilt, and astigmatism. Ophthalmic Res 17:54, 1985 150. Drews RC: Quality control and changing indications for lens implantation. Ophthalmology 90:301, 1983 151. Olson RJ, Waters SW: The clinical use, accuracy, and reliability of the Veri-Vu Lensometer. Arch Ophthalmol 98:2060, 1980 152. Cameron JO, Lane SS, Lindstrom RL: The importance of intraocular lens inspection prior to implantation. Ophthalmic Surg 20:250, 1989 153. Ohara K, Okada K, Akahoshi T: Surface quality of intraocular lenses. J Cataract Refract Surg 15:105, 1989 154. Rosner M, Sharir M, Blumenthal M: Letter: Optical aberrations from a well-centered intraocular lens implant. Am J Ophthalmol 101:117, 1986 155. Brems RN, Apple OF, Pfeffer BR et al: Posterior chamber intraocular lenses in a series of 75 autopsy eyes: Part
III. Correlation of positioning holes and optic edges with the papillary
aperture and visual axis.J Cataract Refract Surg 12:367, 1986 156. McDonnell PJ, Spalton DJ, Falcon MG: Decentration of the posterior chamber lens implant: The effect of optic
size on the incidence of visual aberrations. Eye 4:132, 1990 157. Schechter RJ: Pupillary peaking with exposure of an intra-ocular lens positioning hole
corrected by Nd:YAG laser treatment. J Cataract Refract Surg 14:86, 1988 158. Ohara K, Abe Kuniomi A: Role of positioning holes in intraocular lens glare. J Cataract Refract Surg 15:647, 1989 159. Apple DJ, Lichtenstein SB, Heerlein K et al: Visual aberrations caused by optic components of posterior chamber intraocular
lenses. J Cataract Refract Surg 13:431, 1987 160. Landry RA: Unwanted optical effects caused by intraocular lens positioning holes. J Cataract Refract Surg 13:421, 1987 161. Friedberg HL, Kline OR, Friedberg AH: Comparison of the unwanted optical images produced by 6 mm and 7 mm intraocular
lenses. J Cataract Refract Surg 15:541, 1989 162. Sharir M, Rosner M, Blumenthal M: Choosing an intraocular lens for patients with large pupils. J Cataract Refract Surg 14:88, 1988 163. Halpern BL, Gallagher SP: Refractive error consequence of reversed-optic AMO SI-40NB lens. Ophthalmology 106:901, 1999 164. Downing JE, Sayano RR: Change in effective power of posterior chamber lenses placed with the piano
surface anterior. Am Intraocular Implant Soc J 9:297, 1983 165. Fechner PU, Barth R: Effect on the retina of an air cushion in the anterior chamber and coaxial
illumination. Am J Ophthalmol 96:600, 1983 166. DeJuan E Jr, McCuen BW, Tiedeman J: Optical hazards of intraocular lenses
during vitreous surgery. Am J Ophthalmol 97:386, 1984 (Letter) 167. Lim JI, Kupperman BD, Gwon A et al: Vitreoretinal surgery through multifocal intraocular lenses compared with
monofocal intraocular lenses in fluid-filled and air-filled rabbit
eyes. Ophthalmology 107:1083, 2000 168. Simcoe CW: Ridley revisited: Anatomic and lens design considerations in
posterior chamber pseudophakia. InEmery JM, Jacobson AC (eds): Current
Concepts in Cataract Surgery: Selected Proceedings of the Sixth Biennial
Cataract Surgical Congress. St Louis: CV Mosby, 1980:133–143 169. Lindstrom RL, Harris WS: Management of the posterior capsule following posterior chamber lens implantation. Am Intraocular Implant Soc J 6:255, 1980 170. Choyce DP: The theoretical ideal for an artificial lens implant to correct aphakia. Trans Ophthalmol Soc UK 97:94, 1977 171. Jalie M: The design of intraocular lenses. Br J Physiol Optics 32:1, 1978 172. Smith G, Cheng-Wan L: The spherical aberration of intraocular lenses. Ophthalmic Physiol Opt 8:287, 1988 173. Atchison DA: Optical design of poly(methyl methacrylate) intraocular lenses. J Cataract Refract Surg 16:178, 1990 174. Wang G, Pomerantzeff O: Obtaining a high-quality retinal image with a biconvex intraocular lens. Am J Ophthalmol 94:87, 1982 175. Holladay JT, Bishop JE, Prager TC et al: The ideal intraocular lens. CLAO J 9:15, 1983 176. Pearlstein CS, Lane SS, Lindstrom RL: The incidence of secondary posterior capsulotomy in convex-posterior vs. convex-anterior
posterior chamber intraocular lenses.J Cataract Refract Surg 14:578, 1988 177. McCartney DL, Miller KM, Stark WJ et al: Intraocular lens style and refraction in eyes treated with silicone oil. Arch Ophthalmol 105:1385, 1987 178. McCuen BW, Klombers L: The choice of posterior chamber intraocular lens style in patients with
diabetic retinopathy. Arch Ophthalmol 108:1376, 1990 179. Pomerantzeff O. Pankratov MM, Want G: Calculation of an IOL from the wide-angle optical model of the eye. Am Intraocular Implant Soc J 11:37, 1985 180. Duke-Elder S: System of Ophthalmology. Vol 4: Physiology of the Eye and
of Vision. St Louis: CV Mosby, 1968:461 181. Miller D: Intraocular lenses. Ann Ophthalmol 13:541, 1981 182. Zigman S: Tinting of intraocular lens implants. Arch Ophthalmol 100:998, 1982 183. Miller D, Lazenby GW: Glare sensitivity in corrected aphakes. Ophthalmol Surg 8:54, 1977 184. Abraham FA, Levartovsky S, Blumenthal M: Visual thresholds in aphakia and pseudophakia. J Cataract Refract Surg 15:432, 1989 185. Aarnisaio E: Unilateral intraocular lens: Matching brightness and colour perception
against the phakic fellow eye. Acta Ophthalmol 66:104, 1988 186. Jay JL, Gautam VB, Allan D: Colour perception in pseudophakia. Br J Ophthalmol 66:658, 1982 187. Hess RF, Woo GO, White PD: Contrast attenuation characteristics of iris clipped intraocular lens implants
in situ. Br J Ophthalmol 69:129,1985 188. Van der Heijde GL, Weber J, Boukes R: Effects of stray light on visual acuity in pseudophakia. Doc Ophthalmol 59:81, 1985 189. Kirkness CM, Weale RA: Does light pose a hazard to the macula in aphakia? Trans Ophthalmol Soc UK 104:699, 1985 190. Thomas M, Fishman GA, Vander Meulan D: Spectral transmission characteristics of intraocular and aphakic contact
lenses. Arch Ophthalmol 101:92, 1983 191. Werner JS, Hardenbergh FE: Spectral sensitivity of the pseudophakic eye. Arch Ophthalmol 101:758, 1983 192. Mainster MA: Spectral transmittance of intraocular lenses and retinal damage from intense
light sources. Am J Ophthalmol 85:167, 1978 193. Mainster MA: Light and macular degeneration: A biophysical and clinical perspective. Eye 1:304, 1987 194. Peyman GA, Zak R, Sloane H: Ultraviolet-absorbingpseudophakos: An effcacy study. Am Intraocular ImplantSoc J 9:161, 1983 195. Peyman GA, Sloan HD, Lim J: Ultraviolet light-absorbing pseudophakos. Am Intraocular Implant Soc J 8:357, 1982 196. Lindstrom RL, Doddi N: Ultraviolet light absorption in intraocular lenses. J Cataract Refract Surg 12:285, 1986 197. Kraff MC, Sanders DR, Jampol LM et al: Effect of an ultraviolet-filtering intraocular lens on cystoid macular
edema. Ophthalmology 92:366, 1985 198. Komatsu M, Kanagami S, Shimizu K: Ultraviolet-absorbing intraocular lens versus non-UV-absorbing intraocular
lens: Comparison of angiographic cystoid macular edema. J Cataract Refract Surg 15:654, 1989 199. Jampol LM, Kraff MC, Sanders DR et al: Near-UV radiation from the operating microscope and pseudophakic cystoid
macular edema. Arch Ophthalmol 103:28, 1985 200. Lawrence HM, Reynolds TR: Erythropsial phototoxicity associated with nonultraviolet-filtering intraocular
lenses. J Cataract Refract Surg 15:569, 1989 201. Werner JS, Steele VG, Pfoff DS: Loss of human photoreceptor sensitivity associated with chronic exposure
to ultraviolet radiation. Ophthalmology 96:1552, 1989 202. Mainster MA: The spectra, classification, and rationale of ultraviolet-protective intraocular
lenses. Am J Ophthalmol 102:727, 1986 203. Clayman HM: Ultraviolet-absorbing chromophores: Chemical and ultraviolet transmission
characteristics. J Cataract Refract Surg 12:529, 1986 204. Miller SA, James RH: Variables associated with ultraviolet transmittance measurements of intraocular
lenses. Am J Ophthalmol 106:256, 1988 205. Farbowitz MA, Zabriskie NA, Crandall AS et al: Visual complaints associated with the AcrySof acrylic intraocular lens. J Cataract Refract Surg 26:1339, 2000 206. Stamler JF, Blodi CR, Verdier D et al: Microscope light induced maculopathy in combined penetrating keratoplasty, extracapsular
cataract extraction, and intraocular lens implantation. Ophthalmology 95:1142, 1988 207. Jaffe GJ, Wood IS: Retinal phototoxicity from the operating microscope: A
protective effect by the fovea. Arch Ophthalmol 106:445, 1988 (Letter) 208. Donzis PB, DeBartolo DF, Lewen RM et al: Light-induced maculopathy and cystoid macular edema. J Cataract Refract Surg 14:84, 1988 209. Hupp SL: Delayed, incomplete recovery of macular function after photic
retinal damage associated with extracapsular cataract extraction and posterior
lens insertion. Arch Ophthalmol 105:1022, 1987 (Letter) 210. Cech JM, Choromokos EA, Sanitato JA: Light-induced maculopathy following
penetrating keratoplasty and lens implantation. Arch Ophthalmol 105:751, 1987 (Letter) 211. Irvine AR, Wood I, Morris BW: Retinal damage from the illumination of the operating microscope. Arch Ophthalmol 102:1358, 1984 212. Colvard DM: Operating microscope light-induced retinal injury: Mechanisms, clinical
manifestations, and preventive measures. Am Intraocular Implant Soc J 10:438, 1984 213. Robertson DM, McLaren JW: Photic retinopathy from the operating room microscope: Study with filters. Arch Ophthalmol 107:373, 1989 214. Jaffe GJ, Irvine AR, Wood IS et al: Retinal phototoxicity from the operating microscope: The role of inspired
oxygen. Ophthalmology 95:1130, 1988 215. McDonald HR, Irvine AR: Light-induced maculopathy from the operating microscope in extracapsular
cataract extraction and intraocular lens implantation. Ophthalmology 90:945, 1983 216. Johnson R, Schatz H, McDonald HR: Photic maculopathy: Early angiographic
and ophthalmoscopic findings and late development of choroidal folds. Arch
Ophthalmol 105:1633, 1987 (Letter) 217. Leonardy NJ, Dabbs CK, Sternberg P Jr: Subretinal neovascularization after
operating microscope burn. Am J Ophthalmol 109:224, 1990 (Letter) 218. McDonald HR, Harris MJ: Operating microscope-induced retinal phototoxicity during pars plane vitrectomy. Arch Ophthalmol 106:521, 1988 219. DeLaey JJ, De Wachter A, Van Oye R et al: Retinal phototrauma during intra-ocular lens-implantation. Int Ophthalmol 7:109, 1984 220. Brod RD, Ball SF, Packer AJ: A model for predicting the site of paraxial retinal lesions secondary to “coaxial” operating microscope illumination systems. Am J Ophthalmol 104:516, 1987 221. Brod RD, Olsen KR, Ball SF et al: The site of operating microscope light-induced injury on the human retina. Am J Ophthalmol 107:390, 1989 222. Khwarg SO, Linstone FA, Daniels SA et al: Incidence, risk factors, and morphology in operating microscope light retinopathy. Am J Ophthalmol 103:255, 1987 223. Girard LJ, Friedman B, Moore CD et al: Intraocular implants and contact lenses: A comparison of the visual functions
of monocularly aphakic patients treated by pupillary intraocular
lens implants and corneal contact lenses. Arch Ophthalmol 68:762, 1962 224. Binkhorst CD, Gobin MH, Leonard PAM: Posttraumatic artificial lens implants (pseudophakoi) in children. Br J Ophthalmol 53:518, 1969 225. Drews RC: A practical approach to lens implant power. Am Intraocular Implant Soc Newslett 1:50, 1975 226. Maltzman BA, Cinotti DJ, Horan CA et al: Posterior chamber implants and postoperative refractive astigmatism. CLAO J 9:229, 1983 227. Baltzman BA, Haupt EJ, Capiello L et al: Anterior chamber implants and postoperative astigmatism. CLAO J 12:32, 1986 228. Lakshminarayanan V, Enoch JM, Raasch T et al: Refractive changes induced by intraocular lens tilt and longitudinal displacement. Arch Ophthalmol 104:90, 1986 229. Binkhorst RD: The cause of excessive astigmatism with intraocular lens implants. Ophthalmology 86:672, 1979 230. Jolson AS, Seidl FJ: Postoperative astigmatism inducedby intraocular lens tilt. Am Intraocular Implant Soc J 10:213, 1984 231. Korynta J, Bok J, Cendelin J: Changes in refraction induced by changes in intraocular lens position. J Refract Corneal Surg 10:556, 1994 232. Korynta J, Bok J, Cendelin J et al: Computer modeling of visual impairment caused by intraocular lens misalignment. J Cataract Refract Surg 25:100, 1999 233. Sasaki K, Sakamoto Y, Shibata T et al: Measurement of postoperative intraocular lens tilting and decentration
using Scheimpflug images. J Cataract Refract Surg 15:454, 1989 234. Phillips P. Rosskothen HD, Perez-Emmanuelli J et al: Measurement of intraocular lens decentration and tilt in vivo. J Cataract Refract Surg 14:129, 1988 235. Guyton DL, Uozato H, Wisnicki HJ: Rapid determination of intraocular lens tilt and decentration through the
undilated pupil. Ophthalmology 97:1259, 1990 236. Hansen SO, Tetz MR, Solomon KD et al: Decentration of flexible loop posterior chamber intraocular lenses in a
series of 222 postmortem eyes. Ophthalmology 95:344, 1988 237. McDonnell PJ, Champion R, Green WR: Location and composition of haptics of posterior chamber intraocular lenses. Ophthalmology 94:136, 1987 238. Abdel-Hakim AS: Corneal astigmatism induced by oversized rigid anterior chamber implants. Am Intraocular Implant Soc J 11:474, 1985 239. Hamed LM, Helveston EM, Ellis FD: Persistent binocular diplopia after cataract surgery. Am J Ophthalmol 103:741, 1987 240. Boerner CF, Thrasher BH: Results of monovision correction in bilateral pseudophakes. Am Intraocular Implant Soc J 10:49, 1984 241. Wilson DG: Achieving a predictable refraction following IOL implantation. Cataract 1:7, 1984 242. Schipper I: Anisophoria after implantation of an intraocular lens. Am Intraocular Implant Soc J 11:290, 1985 243. Binkhorst CD, Gobin MH: Injuries to the eye with lens opacities in young children. Ophthalmologica 148:169, 1964 244. Wang G. Pomerantzeff O: Iseikonia and accommodation in the fellow eye in monocular IOL implantation. Am Intraocular Implant Soc J 9:441, 1983 245. Shoch D: Cataracts and macular degeneration. Am J Ophthalmol 88:499, 1979 246. Stark WJ, Kracher GP, Cowan CL et al: Extended-wear contact lenses and intraocular lenses for aphakic correction. Am J Ophthalmol 88:535, 1979 247. Faye EE: Clinical Low Vision. Boston: Little, Brown, 1976:228 248. Newsome DA, Stark WJ, Maumenee IH: Cataract extraction and intraocular lens implantation in patients with
retinitis pigmentosa or Usher's syndrome. Arch Ophthalmol 104:852, 1986 249. Moore CR: A scan accuracy. In Emery JM (ed): Current Concepts in Cataract
Surgery. St Louis: CV Mosby, 1978:184–185 250. Tennant JL: Calculation of the power of the Peter Choyce anterior chamber
intraocular lens. In Emery JM (ed): Current Concepts in Cataract Surgery. St
Louis: CV Mosby, 1978:194 251. Troutman RC: Artiphakia and aniseikonia. Am J Ophthalmol 56:602, 1963 252. Katsumi O, Miyanaga Y, Hirose T et al: Binocular function in unilateral aphakia: Correlation with aniseikonia
andstereoacuity. Ophthalmology 95:1088, 1988 253. Crone RA, Leuridan OMA: Unilateral aphakia and tolerance of aniseikonia. Ophthalmologica 171:258, 1975 254. Burian HM: Optics: Fusion in unilateral aphakia. Trans Am Acad Ophthalmol Otolaryngol 66:285, 1962 255. Highman VN: Stereopsis and aniseikonia in uniocular aphakia. Br J Ophthalmol 61:30, 1977 256. Troutman RC: Artiphakia and aniseikonia. Trans Am Acad Ophthalmol Otolaryngol 60:590, 1962 257. Troutman RC: Correction of unilateral aphakia: The use of intraocular lens implants. Arch Ophthalmol 68:861, 1962 258. Weis DR: Long-term results wearing hard contact lenses in monocular aphakia. Ophthalmology 89:1003, 1982 259. Katsumi O, Tanino T, Hirose T: Effects of aniseikonia on binocular fusion. Invest Ophthalmol Vis Sci 27:601, 1986 260. Miyake S, Awaya S, Miyake K: Aniseikonia in patients with a unilateral artificial lens, measured with
Aulhorn's phase difference haploscope. Am Intraocular ImplantSoc J 7:36, 1981 261. Nolan J, Hawkswell A, Becket S: Fusion in aphakia. Proceedings of the Third
International Orthoptic Congress, Boston, 1975 262. Girard LJ: Ultrasonic Fragmentation for Intraocular Surgery. St Louis: CV
Mosby, 1979:194 263. Choyce P: Intraocular Lenses and Implants. London: Lewis, 1964 264. Saunders RA, Ellis FD: Empirical fitting of hard contact lenses in infants and young children. Ophthalmology 88:127, 1981 265. Percival SPB, Yousef KM: Treatment of uniocular aphakia: A comparison of iris clip lenses with hard
corneal contact lenses. Br J Ophthalmol 60:642, 1976 266. Isomura Y, Awaya S: Studies on aniseikonia and binocular fusion with special reference to stereoacuity [English
abstract and tables]. Nippon Ganka Gakkai Zasshi (Acta Soc Ophthalmol Jpn) 84:1619, 1980 267. Galin MA, Baras I: Stereoscopic acuity measurement in aphakia. Am J Ophthalmol 86:825, 1978 268. Hales RH: Silicone extended-wear contact lenses in aphakic patients: A comparison
with intraocular lenses over four years of continuous use. Contact Lens 7:219, 1981 269. Stein HA: Aphakia: Selection of patients for contact lenses, intraocular lenses or
spectacles—review of 1,000 cataract operations. Contact Lens 7:210, 1981 270. Binkhorst CD, Gobin MH: Pseudophakia after lens injury in children. Ophthalmologica 154:81, 1967 271. van Balen AThM: Binkhorst's method of implication of Pseudophakia in unilateral traumatic
cataract. Ophthalmologica 165:490, 1972 272. Daniel R: An evaluation of contact lenses in unilateral post-traumatic aphakic children. Contact Lens 4:19, 1974 273. Bierlaagh JJM, van der Wee A, Kats A et al: Techniques and perspectives
of lens implants (pseudophakoi) in children. Proceedings of Second International
Orthoptics Congress, Amsterdam (International Congress Series
No. 245). Amsterdam: Excerpta Medica, 1971 274. Dulaney DD, Freeman LW: Shearing-style vs. Platina-style IOLs. Am Intraocular
Implant Soc J 6:369, 1980 (Letter) 275. Harris M: Correction of pediatric aphakia with silicone contact lenses. CLAO J 11:343, 1985 276. Rogers GL, Tishler CL, Tsou BH et al: Visual acuities in infants with congenital cataracts operated on prior
to 6 months of age. Arch Ophthalmol 99:999, 1981 277. Ben-Ezra D, Paez JH: Congenital cataract and intraocular lenses: Am J Ophthalmol 96:311, 1983 278. Hiles DA: Intraocular lenses: Visual rehabilitation of aphakic children. Surv Ophthalmol 34:371, 1990 279. Hiles DA: Intraocular lens implantation in children with monocular cataracts 1974-1983. Ophthalmology 91:1231, 1984 280. Aron JJ, Aron-Rosa D: Intraocular lens implantation in unilateral congenital cataract: A preliminary
report. Am Intraocular Implant Soc J 9:306, 1983 281. Wilson ME, Bluestein EC, Want XH: Current trends in the use of intraocular lenses in children. J Cataract Refract Surg 20:579, 1994 282. Wilson ME: Intraocular lens implantation: Has it become the standard of
care for children? Ophthalmology 103:1719, 1966 (Editorial) 283. Beller R. Hoyt C, Marg E et al: Good visual function after neonatal surgery for congenital monocular cataracts. Am J Ophthalmol 91:559, 1981 284. Gordon RA, Donzis PB: Refractive development of the human eye. Arch Ophthalmol 103:785, 1985 285. Stark WJ, Taylor HR, Michels RG et al: Management of congenital cataracts. Trans Am Acad Ophthalmol Otolaryngol 86:1571, 1979 286. Rogers GL: Extended wear silicone contact lenses in children with cataracts. Ophthalmology 87:867, 1980 287. Del Monte MA: Diagnosis and management of congenital and developmental cataracts. Ophthalmol Clin North Am 3:205, 1990 288. Vila-Coro AA, Mazow ML: Initiation of amblyopia treatment in monocular
congenital cataracts. Arch Ophthalmol 107:1113, 1989 (Letter) 289. Drummond GR, Scott WE, Keech RV: Management of monocular congenital cataracts. Arch Ophthalmol 107:45, 1989 290. Levin AV, Edmonds SA, Nelson LB et al: Extended-wear contact lenses for the treatment of pediatric aphakia. Ophthalmology 95:1107, 1988 291. Amaya LG, Speedwell L, Taylor D: Contact lenses for infant aphakia. Br J Ophthalmol 74:150, 1990 292. Hiles DA, Hered RW: Modern intraocular lens implants in children with new age limitations. J Cataract Refract Surg 13:493, 1987 293. Sinsky RM, Karel F, Ri ED: Management of cataracts in children. J Cataract Refract Surg 15:196, 1989 294. Zwaan J, Mullaney PB, Awad A et al: Pediatric intraocular lens implantation. Ophthalmology 105:112, 1998 295. Cavallaro BE, Madigan WP, O'Hara MA et al: Posterior chamber intraocular lens use in children. J Pediatr Ophthalmol Strabismus 35:254, 1998 296. Zubcov AA, Stahl E, Rossillion B et al: Stereopsis after primary in-the-bag posterior chamber implantation in children. JAAPOS 3:227, 1999 297. Green DG, Powers MK, Banks MS: Depth of focus, eye size, and visual acuity. Vis Res 20:827, 1980 298. Powers MK, Dobson V: Effect of focus on visual acuity of human infants. Vis Res 22:521, 1982 299. Enyedi LB, Peterseim MW, Freedman SF et al: Refractive changes after pediatric intraocular lens implantation. Am J Ophthalmol 126:772, 1998 300. Basti S, Jensen A, Greenwald MJ: Refractive changes after pediatric intraocular
lens implantation. Am J Ophthalmol 128:394, 1999 (Letter) 301. Dahan E, Drusedau MUH: Choice of lens and dioptric power in pediatric pseudophakia. J Cataract Refract Surg 23(Suppl 1):618, 1997 302. Lambert SR, Buckley EG, Plager DA et al: Unilateral intraocular lens implantation during the first six months of
life. JAAPOS 3:344, 1999 303. Hutchinson AK, Wilson ME, Saunders RA: Outcomes and ocular growth rates after intraocular lens implantation in
the first 2 years of life. J Cataract Refract Surg 24:846, 1998 304. Knight-Nanan D, O'Keefe M, Bowell R: Outcomes and complications of intraocular lenses in children with cataract. J Cataract Surg 22:730, 1996 305. Lambert SR, Fernandes A, Drews-Botsch C et al: Pseudophakia retards axial elongation in neonatal monkey eyes. Invest Ophthalmol Vis Sci 37:451, 1996 306. Badr IA, Hussain HM, Jabak M et al: Extracapsular cataract extraction with or without posterior chamber intraocular
lens in eyes with cataract and high myopia. Ophthalmology 102:1139, 1995 307. Ball JL, Brittain GPH: Zero or low-power IOL implantation rather than planned
aphakia. J Cataract Refract Surg 26:1437, 2000 (Letter) 308. Kohnen S, Brauweiler P: First results of cataract surgery and implantation of negative power intraocular
lenses in highly myopic eyes. J Cataract Refract Surg 22:416, 1996 309. Duke-Elder S, Abrams D: System of Ophthalmology, Vol 5. Ophthalmic Optics
and Refraction. St. Louis: CV Mosby, 1970:71 310. Christman EH: Correction of aniseikonia in monocular aphakia. Arch Ophthalmol 85:148, 1971 311. Huber C: Planned myopic astigmatism as a substitute for accommodation in pseudophakia. Am Intraocular Implant Soc J 7:244, 1981 |