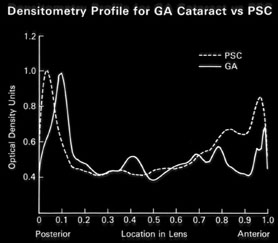
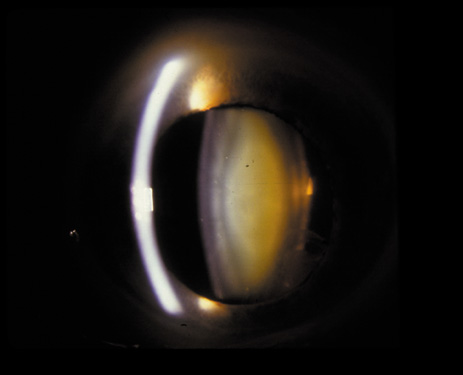
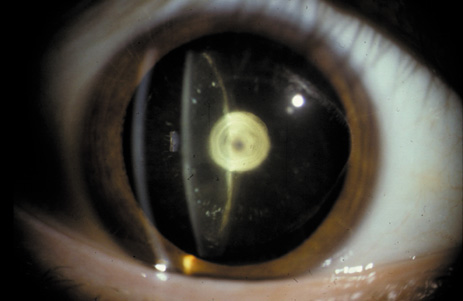
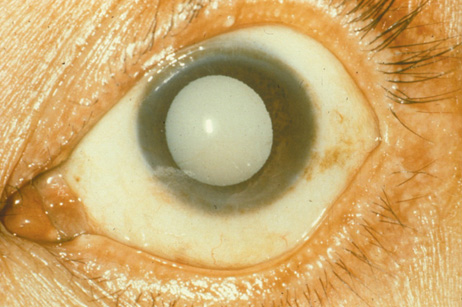
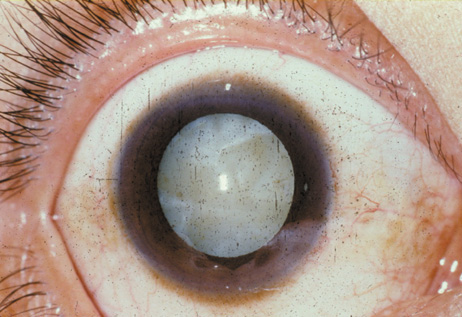
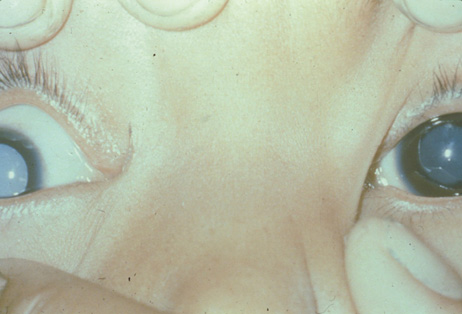
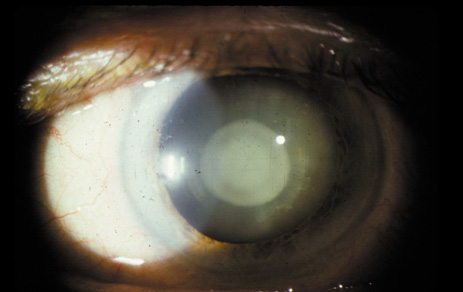
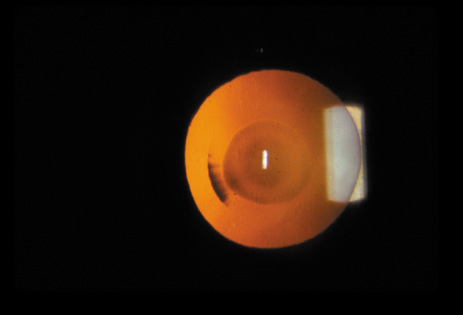
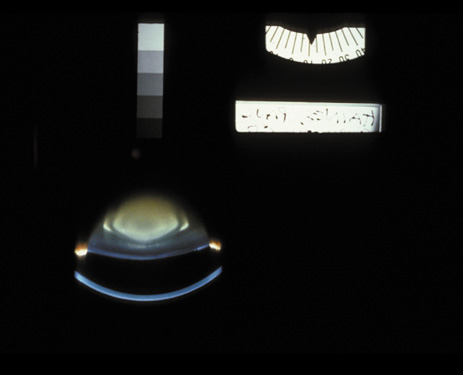
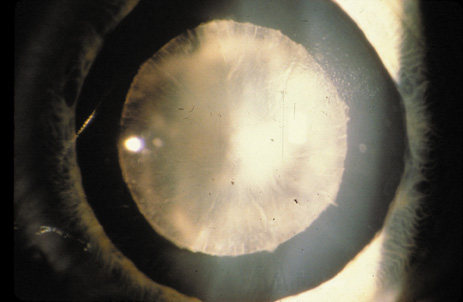
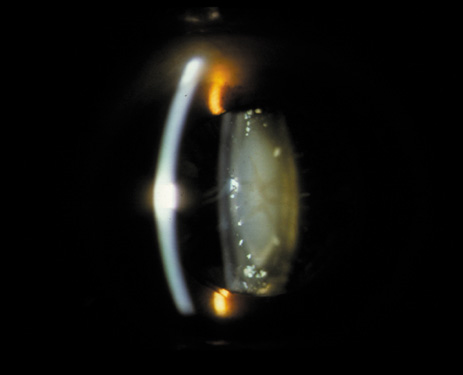
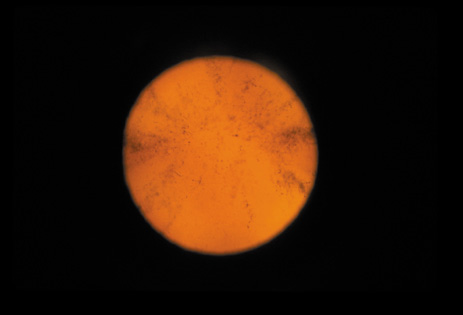
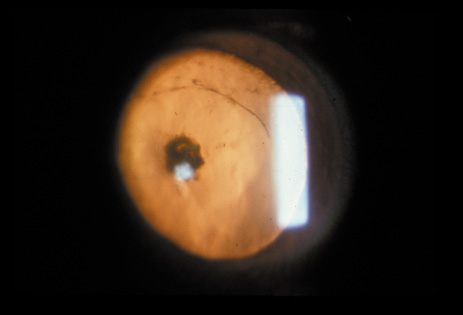
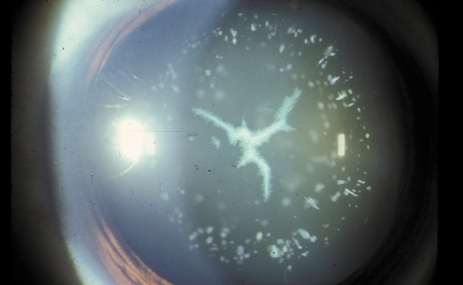
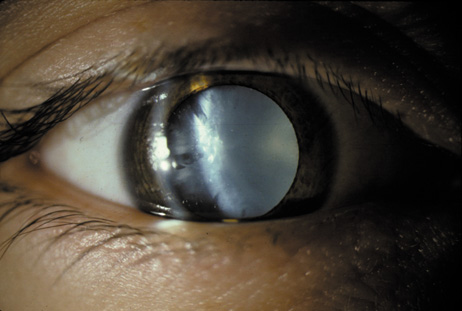
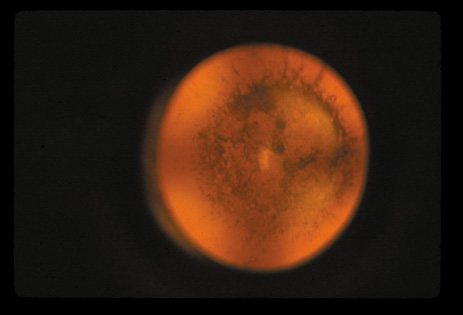
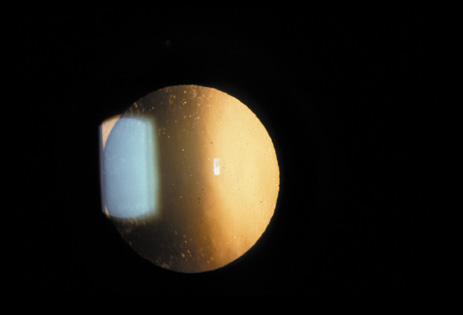
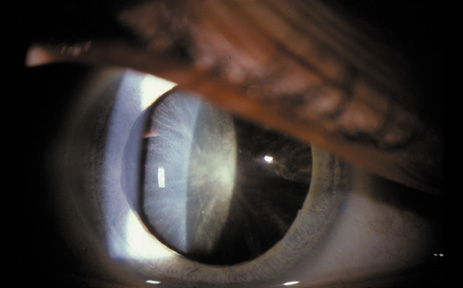
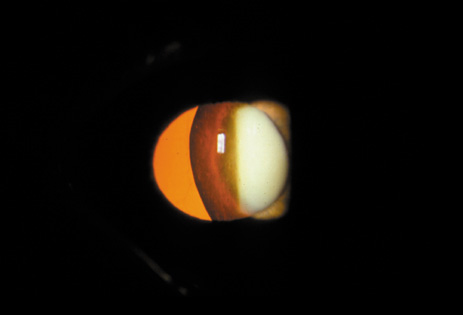
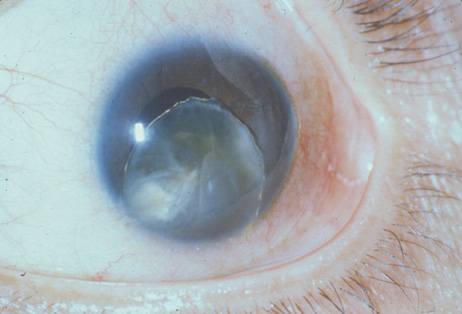
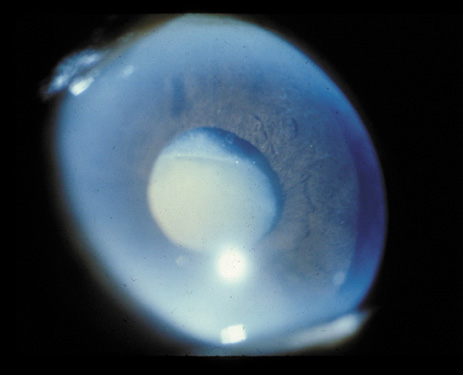
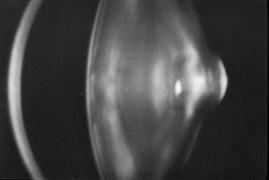
1. Congdon N, Vingerling JR, Klein BEK, et al: The prevalence of cataract and pseudophakia/aphakia among adults in
the United States. Arch Ophthalmol 122:487–494, 2004 2. Duke-Elder S: System of ophthalmology. Vol. 11. St. Louis: CV Mosby, 1969:63 3. Benedek GB, Chylack LT Jr, Libondi T, et al: Quantitative detection of the molecular changes associated with early cataractogenesis
in the living human lens using quasielastic light scattering. Curr Eye Res 6:1421, 1987 4. Congdon N, O'Colmain B, Klaver CC, et al: Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol 122:477–485, 2004 5. Thylefors B, Negrel AD, Pararajasegaram R, Dadzie KY: Global data on blindness. Bull World Health Org 73:115–121, 1995 6. Congdon NG: Prevention strategies for age related cataract: present limitations and
future possibilities. Br J Ophthalmol 85:516–520, 2001 7. Baltussen R, Sylla M, Mariotti SP: Cost-effectiveness analysis of cataract surgery: a global and regional
analysis. Bull World Health Org 82:338–345, 2004 8. Busbee BG, Brown GC, Brown MM: Cost-effectiveness of ocular interventions. Curr Opin Ophthalmol 14:132–138, 2003 9. Steinberg EP, Javitt JC, Sharkey PD, et al: The content and cost of cataract surgery. Arch Ophthalmol 111:1041, 1993 10. Chylack LT Jr, Leske MC, McCarthy D, et al: Lens opacities classification system II (LOCS II). Arch Ophthalmol 107:991, 1989 11. Chylack LT Jr, Wolfe JK, Singer DM, et al: Lens opacities classification system III. Arch Ophthalmol 111:831, 1993 12. Sparrow JM, Bron AJ, Brown NAP, et al: Oxford clinical cataract classification and grading system. Int Ophthalmol 9:207, 1986 13. Klein BEK, Klein R, Linton KLP, et al: Assessment of cataracts from photographs in the Beaver Dam Eye Study. Ophthalmology 97:1428, 1990 14. Taylor HR, West SK: The clinical grading of lens opacities. Aust N Z J Ophthalmol 17:81, 1989 15. Age-Related Eye Disease Study Research Group: The age-related eye disease study (AREDS) system for classifying
cataracts from photographs: AREDS report no. 4. Am J Ophthalmol 131:167–175, 2001 16. Robman LD, McCarty CA, Garrett SK, et al: Comparison of clinical and digital assessment of nuclear optical density. Ophthalmic Res 31:119–126, 1999 17. Garrett SK, Robman LD, McCarty CA, et al: Reproducibility of automatic standard digital analysis of lens opacities. Aust N Z J Ophthalmol 26(Suppl 1):S29–S31, 1998 18. Congdon NG, Taylor HR: Age-related cataract. In: Johnson GJ, Minassian DC, West S, Weale R, (eds.): The Epidemiology of Eye Disease. London, UK: Arnold Publishers, 2003:105–119 19. Kashima K, Trus B, Unser M, et al: Aging studies on normal lens using the Scheimpflug slit lamp camera. Invest Ophthalmol Vis Sci 34:263, 1993 20. Panchapakesan J, Rochtchina E, Mitchell P: Myopic refractive shift caused by incident cataract: the Blue Mountains
Eye Study. Ophthal Epidemiol 10:241–247, 2003 21. Lee KE, Klein BE, Klein R, Wong TY: Changes in refraction over 10 years in an adult population: the Beaver
Dam Eye study. Invest Ophthalmol Vis Sci 43:2566–2567, 2002 22. Horwitz J: Proctor lecture: The function of alpha-crystallin. Invest Ophthalmol Vis Sci 34:10, 1993 23. Van Heyningen R: What happens to the human lens in cataract? In: Spivey B, Henkind P, Lichter P, et al (eds). American Academy of Ophthalmology: Selected Readings in Ophthalmology Companion
Source Manual. Vol. 2. San Francisco: American Academy of Ophthalmology, 1976:112 24. Clark JI, Livesey JC, Steele JE: Phase separation inhibitors and lens transparency. Optom Vis Sci 70:873, 1993 25. Kinoshita JH, Kador P, Datiles M: Aldose reductase in diabetic cataract. JAMA 246:259, 1981 26. Kinoshita JH: Mechanisms initiating cataract formation: Proctor lecture. Invest Ophthalmol 13:713, 1974 27. Kuszak JR: The development of lens sutures. Prog Retinal Eye Res 14:567, 1995 28. Schein O, West S, Mundy B, et al: Cortical lenticular opacification: Distribution and location in a longitudinal
study. Invest Ophthalmol Vis Sci 35:363, 1994 29. West SK, Duncan DD, Munoz B, et al: Sunlight exposure and risk of lens opacities in a population-based study: The
Salisbury Eye Evaluation Project. JAMA 280:714–718, 1998 30. Zigman S, Paxhia T, McDaniel T, et al: Effect of chronic near-ultraviolet radiation on the gray squirrel lens
in vivo. Invest Ophthalmol Vis Sci 32:1723–1732, 1991 31. Lasa S, Datiles M, Podgor M, Magno B: Contrast and glare sensitivity: Association with type and severity of the
cataract. Ophthalmology 99:1045, 1992 32. Lasa S, Podgor M, Datiles M, et al: Glare sensitivity in early cataracts. Br J Ophthalmol 77:489, 1993 33. Datiles MB: Clinical evaluation of cataracts. In: Tasman W, Jaeger EA (eds). Duane's Clinical Ophthalmology. Vol. 1. Philadelphia: JB Lippincott, 1993:6 34. Greiner JV, Chylack LT Jr: Posterior subcapsular cataracts: histopathologic study of steroid-associated
cataracts. Arch Ophthalmol 97:135–144, 1979 35. Harding CV, Chylack LT Jr, Susan SR, et al: Elemental and ultrastructural analysis of specific human lens opacities. Invest Ophthalmol Vis Sci 23:1–13, 1982 36. Chylack LT Jr: Mechanisms of senile cataract formation. Ophthalmology 91:596–602, 1984 37. Wong TY, Klein BE, Klein R, Tomany SC, Lee KE: Refractive errors and incident cataracts: the Beaver Dam Eye Study. Invest Ophthalmol Vis Sci 42:1449–1454, 2001 38. Lim R, Mitchell P, Cumming RG: Refractive associations with cataract: the Blue Mountains Eye Study. Invest Ophthalmol Vis Sci 40:3021–3026, 1999 39. Younan C, Mitchell P, Cumming RG, et al: Myopia and incident cataract and cataract surgery: the Blue Mountains Eye
Study. Invest Ophthalmol Vis Sci 43:3625–3632, 2002 40. Wong TY, Foster PJ, Johnson GJ, Seah SK: Refractive errors, axial ocular dimensions, and age-related cataracts: the
Tanjong Pagar survey. Invest Ophthalmol Vis Sci 44:1479–1485, 2003 41. Chang MA, Congdon NG, Munoz B, et al: The association between myopia and various subtypes of lens opacity: SEE
Project. Ophthalmology (in press)ppyy 42. Fishman GA, Anderson RJ, Lourenco P: Prevalence of posterior subcapsular lens opacities in patients with retinitis
pigmentosa. Br J Ophthalmol 69:263, 1985 43. Fagerholm PP, Philipson BT: Cataract in retinitis pigmentosa: an analysis of cataract surgery results
and pathological lens changes. Acta Ophthalmol (Copenh) 63:50, 1985 44. Kaiser-Kupfer M, Kuwabara T, Uga S, et al: Cataract in gyrate atrophy: clinical and morphologic studies. Invest Ophthalmol Vis Sci 24:432, 1983 45. Kashima K, Datiles M, Trus B, et al: Localization of lens opacities in gyrate atrophy: Image analysis study
with Scheimpflug camera. Folia Ophthalmol Jpn 40:986, 1989 46. Vrensen GF, Willekens B, De Jong PT, et al: Heterogeneity in ultrastructure and elemental composition of perinuclear
lens retrodots. Invest Ophthalmol Vis Sci 35:199, 1994 47. Lovicu FJ, Schulz MW, Hales AM, et al: TGFbeta induces morphological and molecular changes similar to human anterior
subcapsular cataract. Br J Ophthalmol 86:220–226, 2002 48. Wunderlich K, Pech M, Eberle AN, et al: Expression of connective tissue growth factor mRNA in plaques of human
anterior subcapsular cataracts and membranes of posterior capsule opacification. Curr Eye Res 21:627–636, 2000 49. Lovicu FJ, Steven P, Saika S, McAvoy JW: Aberrant lens fiber differentiation in anterior subcapsular cataract formation: a
process dependent on reduced levels of Pax6. Invest Ophthalmol Vis Sci 45:1946–1953, 2004 50. Joo CK, Lee EH, Kim JC, et al: Degeneration and transdifferentiation of human lens epithelial cells in
nuclear and anterior polar cataracts. J Cataract Refract Surg 25:652–658, 1999 51. Marcantonio JM, Syam PP, Liu CS, Duncan G: Epithelial transdifferentiation and cataract in the human lens. Exp Eye Res 77:339–346, 2003 52. Srinivasan Y, Lovicu FJ, Overbeek PA: Lens-specific expression of transforming growth factor beta 1 in transgenic
mice causes anterior subcapsular cataracts. J Clin Invest 101:625–634, 1998 53. Hales AM, Chamberlain CG, McAvoy JW: Susceptibility to TGF-beta2-induced cataract increases with aging in the
rat. Invest Ophthalmol Vis Sci 41:3544–3551, 2000 54. Beethan WP: Atopic cataract. Arch Ophthalmol 24:21, 1940 55. Sasaki K, Kojima M, Nakaizumi H, et al: Early lens changes seen in patients with atopic dermatitis applying image
analysis processing of Scheimpflug and specular microscopic images. Ophthalmologica 212:88–94, 1998 56. Street DA, Javitt JC: National five-year mortality after inpatient cataract extraction. Am J Ophthalmol 113:263, 1992 57. Glynn RJ, Christen WG, Manson JE, et al: Body mass index: an independent predictor of cataract. Arch Ophthalmol 113:1131, 1995 58. Tavani A, Negri E, La Vecchia C: Selected diseases and risk of cataract in women: a case-control study from
northern Italy. Ann Epidemiol 5:234, 1995 59. Leske MC, Chylack LT Jr, Wu SY: Lens opacities case-control study: risk factors for cataract. Arch Ophthalmol 109:244, 1991 60. Robinson GC, Jan JE, Kinnis C: Congenital ocular blindness in children, 1945–1984. Am J Dis Child 141:1321, 1987 61. Wong TY, Klein BE, Klein R, Tomany SC: Relation of ocular trauma to cortical, nuclear, and posterior subcapsular
cataracts: the Beaver Dam Eye Study. Br J Ophthalmol 86:152–155, 2002 62. Nelson LB, Spaeth GL, Nowinski TS, et al: Aniridia: a review. Surv Ophthalmol 28:621, 1984 63. Kaiser-Kupfer MI, Freidlin V, Datiles MB, et al: The association of posterior capsular lens opacities with bilateral acoustic
neuromas in patients with neurofibromatosis type 2. Arch Ophthalmol 107:541, 1989 64. Bouzas EA, Freidlin V, Parry DM, et al: Lens opacities in neurofibromatosis 2: Further significant correlations. Br J Ophthalmol 77:354, 1993 65. Ederer F, Hiller R, Taylor HR: Senile lens changes and diabetes in two population studies. Am J Ophthalmol 91:381–395, 1981 66. Pokupec R, Kalauz M, Turk N, Turk Z: Advanced glycation endproducts in human diabetic and non-diabetic cataractous
lenses. Graefe's Arch Clin Exp Ophthalmol 241:378–384, 2003 67. Kinoshita JH, Datiles MB, Kador PF, Robison WG Jr: Aldose reductase and diabetic eye complications. In: Rifkin H, Porte D (eds). Ellenberg and Rifkin's Diabetes Mellitus: Theory and Practice. 4th
ed. New York: Elsevier, 1990:264 68. Fukushi S, Merola LO, Kinoshita JH: Altering the course of cataracts in diabetic rats. Invest Ophthalmol Vis Sci 19:313, 1980 69. Datiles M, Fukui H: Cataract prevention in diabetic Octodon degus with Pfizer's sorbinil. Curr Eye Res 8:233, 1989 70. Wyman M, Sato S, Akayi Y, et al: The dog as a model for ocular manifestation of high concentration of blood
sugars. J Am Vet Med Assoc 193:1153, 1988 71. Biswas S, Harris F, Singh J, Phoenix D: Role of calpains in diabetes mellitus-induced cataractogenesis: A mini
review. Mol Cell Biochem 261:151–159, 2004 72. Sakamoto-Mizutani K, Fukiage C, Tamada Y, et al: Contribution of ubiquitous calpains to cataractogenesis in the spontaneous
diabetic WBN/Kob rat. Exp Eye Res 75:611–617, 2002 73. Thampi P, Hassan A, Smith JB, Abraham EC: Enhanced C-terminal truncation of alpha A- and alpha B-crystallins, in
diabetic lenses. Invest Ophthalmol Vis Sci 43:3265–3272, 2002 74. Azuma M, Fukiage C, David LL, Shearer TR: Activation of calpain in lens: A review and proposed mechanism. Exp Eye Res 64:529–538, 1997 75. Andersson M, Sjostrand J, Andersson AK, et al: Calpains in lens epithelium from patients with cataract. Exp Eye Res 59:359–364, 1994 76. Hedge KR, Varma SD: Morphogenetic and apoptotic changes in diabetic cataract: Prevention by
pyruvate. Mol Cell Biochem 262:233–237, 2004 77. Beutler E, Matsumono F, Kuhl W, et al: Galactokinase deficiency as a cause of cataracts. N Engl J Med 288:1203, 1973 78. Holton JB, Walter JH, Tyfield LA: Galactosemia. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds). The Metabolic and Molecular Bases of Inherited Disease. 8th ed. New York: McGraw-Hill, 2001:1553–1583 79. Bosch AM, Bakker HD, Van Gennip AH, et al: Clinical features of galactokinase deficiency: A review of the literature. J Inherit Metab Disord 25:629–634, 2002 80. Okano Y, Asada M, Fujimoto A, et al: A genetic factor for age-related cataract: identification and characterization
of a novel galactokinase variant, “Osaka,” in Asians. Am J Hum Genet 68:1036–1042, 2001 81. Datiles M, Fukui H, Kuwabara T, Kinoshita JH: Galactose cataract prevention with sorbinil, an aldose reductase inhibitor: a
light microscopic study. Invest Ophthalmol Vis Sci 22:174, 1982 82. Skalka HW, Prchal JT: Presenile cataract formation and decreased activity of galactosemic enzymes. Arch Ophthalmol 98:269–273, 1980 83. Stevens RE, Datiles MB, Srivastava SK, et al: Idiopathic presenile cataract formation and galactosemia. Br J Ophthalmol 73:48–51, 1989 84. Karas N, Gobec L, Pfeifer V, et al: Mutations in galactose-1-phosphate uridyltransferase gene in patients with
idiopathic presenile cataract. J Inherit Metab Dis 26:699–704, 2003 85. Lukac-Bajalo J: Idiopathic presenile cataracts and galactosemia. Adv Clin Path 1(suppl 1):L5, 1997 86. Podskarbi T, Kohlmetz T, Gathof BS, et al: Molecular characterization of Duarte-1 and Duarte-2 variants of galactose-1-phosphate
uridyltransferase J Inherit Metab Dis 19:638–644, 1996 87. Simonelli F, Nesti A, Picardi A, et al: Cataract formation in patients with lactose and galactose disorders. Dev Ophthalmol 17:82–86, 1989 88. Gardner RJ, Brown N: Lowe's syndrome: identification of carriers by lens examination. J Med Genet 13:449, 1976 89. Hedges TR Jr, Hedges TR 3rd: Wilson's disease (hepatolenticular degeneration). In: Gold DH, Weingeist TA (eds). The Eye in Systemic Disease. Philadelphia: JB Lippincott, 1990:390 90. Sher NA, Letson RD, Desnick RJ: The ocular manifestations in Fabry's disease. Arch Ophthalmol 97:671, 1979 91. Ramsay RC: Homocystinuria. In: Gold DH, Weingeist TA (eds). The Eye in Systemic Disease. Philadelphia: JB Lippincott, 1990:319 92. Morris DA: Cataracts and systemic disease. In: Duane TD, Jaeger EA (eds). Duane's Clinical Ophthalmology. Vol. 5. Philadelphia: JB Lippincott, 1993:8– 13 93. Claridge KG, Gibberd FB, Sidey MC: Refsum disease: the presentation and ophthalmic aspects of Refsum disease
in a series of 23 patients. Eye 6:371, 1992 94. Grondalski SJ, Bennett GR: Alport's syndrome: review and case report. Optom Vis Sci 66:396, 1989 95. Mintz-Hittner H, Kretzer FL: Conradi's syndrome. In: Gold DH, Weingeist TA (eds). The Eye in Systemic Disease. Philadelphia: JB Lippincott, 96. Raitta C, Karli P: Ocular findings in myotonic dystrophy. Ann Ophthalmol 14:647, 1982 97. Cross HE, Jensen AD: Ocular manifestations in the Marfan syndrome and homocystinuria. Am J Ophthalmol 75:405–420, 1973 98. Hutchinson S, Furger A, Halliday D, et al: Allelic variation in normal human FBN1 expression in a family with Marfan
syndrome: a potential modifier of phenotype? Hum Mol Genet 12:2269–2276, 2003 99. Hamod A, Moodie D, Clark B, Traboulsi EI: Presenting signs and clinical diagnosis in individuals referred to rule
out Marfan syndrome. Ophthalmic Genet 24:35–39, 2003 100. Sachdev NH, Di Girolamo N, McCluskey PJ, et al: Lens dislocation in Marfan syndrome: Potential role of matrix metalloproteinases
in fibrillin degradation. Arch Ophthalmol 120:833–835, 2002 101. Ashworth JL, Murphy G, Rock MJ, et al: Fibrillin degradation by matrix metalloproteinases: implications for connective
tissue remodelling. Biochem J 340:171–181, 1999 102. Sachdev N, Wakefield D, Coroneo MT: Lens dislocation in Marfan syndrome and UV-B light exposure. Arch Ophthalmol 121:585–585, 2003 103. Weill G: Ectopie du cristallin et malformations generales. Ann Ocul 169:21–44, 1932 104. Marchesani O: Brachydaktylie und angeborene kugellines als systemerkrankung. Klin Mibl Augenheilkd 103:392–406, 1939 105. Faivre L, Dollfus H, Lyonnet S, et al: Clinical homogeneity and genetic heterogeneity in Weill-Marchesani syndrome. Am J Med Genet 123A:204–207, 2003 106. Veloes A, Hermia JP, Galand A, et al: Glaucoma-lens ectopia microsherophakia-stiffness-shortness (GEM-SS) syndrome: A
dominant disease with manifestations of Weill-Marchesani
syndromes. Am J Med Genet 44:48–51, 1992 107. Kloepfer HW, Rosenthal JW: Possible genetic carriers in the spherophakia-brachymorphia syndrome. Am J Hum Genet 7:398–425, 1955 108. Wirtz MK, Samples JR, Kramer PL, et al: Weill-Marchesani syndrome–possible linkage of the autosomal dominant
form to 15q21.1. Am J Med Genet 65:68–75, 1996 109. Faivre L, Gorlin RJ, Wirtz MK, et al: In frame fibrillin-1 gene deletion in autosomal dominant Weill-Marchesani
syndrome. J Med Genet 40:34–36, 2003 110. Dagoneau N, Benoist-Lasselin C, Huber C, et al: ADAMTS10 mutations in autosomal recessive Weill-Marchesani syndrome. Am J Hum Genet 75:801–806, 2004 111. Tang BL: ADAMTS: a novel family of extracellular matrix proteases. Int J Biochem Cell Biol 33:33–44, 2001 112. Cal S, Obaya AJ, Llamazares M, et al: Cloning, expression analysis and structural characterization of seven novel
human ADAMTS, a family of metalloproteinases with disintegrin and
thrombospondin-1 domains. Gene 283:49–62, 2002 113. Snead MP, Yates JRW: Clinical and molecular genetics of Stickler syndrome. J Med Genet 36:353–359, 1999 114. Seery CM, Pruett RC, Liberfarb RM, Cohen BZ: Distinctive cataract in the Stickler syndrome. Am J Ophthalmol 110:143–148, 1990 115. Lipman RM, Tripathi BJ, Tripathi RC: Cataracts induced by microwave and ionizing radiation. Surv Ophthalmol 33:200, 1988 116. Lydahl E: Infrared radiation and cataract. Acta Ophthalmol 166(suppl):1, 198 117. Cucinotta FA, Manuel FK, Jones J, et al: Space radiation and cataracts in astronauts. Radiat Res 156:460–466, 2001 118. Lett JT, Lee AC, Cox AB: Risks of radiation cataracts from interplanetary space missions. Acta Astronaut 32:739–748, 1994 119. Nicholas JS, Butler GC, Lackland DT, et al: Health among commercial airline pilots. Aviat Space Environ Med 72:821, 2001 120. Urban RC Jr, Cotlier E: Corticosteroid-induced cataracts. Surv Ophthalmol 31:102, 1986 121. Limaye SR, Pillai S, Tina LU: Relationship of steroid dose to degree of posterior subcapsular cataracts
in nephrotic syndrome. Ann Ophthalmol 20:225, 1988 122. Mayman CI, Miller D, Mayman CI: In vitro production of steroid cataract in bovine lens. Part II: Measurement
of sodium-potassium adenosine triphosphatase activity. Acta Ophthalmol 57:1107–1116, 1979 123. Lou MF, Dickerson JE Jr, Garadi R: The role of protein-thiol mixed disulfides in cataractogenesis. Exp Eye Res 50:819–826, 1990 124. Lyu J, Kim JA, Chung SK, et al: The alteration of cadherin in dexamethasone-induced cataract organ-cultured
rat lens. IOVS 44:2034–2040, 2003 125. More MI, Kirsch FP, Rathjein FG: Targeted ablation of NrCAM or ankyrin-B results in disorganized lens fibers
leading to cataract formation. J Cell Biol 154:187–196, 2001 126. Bucala R, Gallati M, Manabe S, et al: A glucocorticoid-lens protein adducts in experimentally induced steroid
cataracts. Exp Eye Res 40:853–863, 1985 127. Hamamichi S, Kosano H, Nakai S, et al: Involvement of hepatic glucocorticoid receptor-mediated functions in steroid-induced
cataract formation. Exp Eye Res 77:575–580, 2003 128. Kosano H, Nishigori H: Steroid induced cataract: other than in whole animal system, in lens culture
system, androgens, estrogens, and progestins as well as glucocorticoids
produce loss of transparency of the lens. Dev Ophthalmol 35:161–168, 2002 129. Reddy VN: Glutathione and its function in the lens–an overview. Exp Eye Res 50:771–778, 1990 130. Spector A: Oxidative stress-induced cataract: mechanism of action. FASEB J 9:1173–1182, 1995 131. Grant WM: Toxicology of the Eye. Springfield: Charles C. Thomas, 1974: 411–710 132. Xu GT, Zigler JS Jr, Lou MF: The possible mechanism of naphthalene cataract in rat and its prevention
by aldose reductase inhibitor (ALO1576). Exp Eye Res 54:63, 1992 133. Tao RV, Takahashi Y, Kador PF: Effect of aldose reductase inhibitors on naphthalene cataract. Invest Ophthalmol Vis Sci 32:1630, 1991 134. Levene RZ: Uniocular miotic therapy. Trans Am Acad Ophthalmol Otolaryngol 79:OP376, 1975 135. Isaac NE, Walker AM, Jick H, Gorman M: Exposure to phenothiazine drugs and risk of cataract. Arch Ophthalmol 109:256, 1991 136. West SK, Valmadrid CT: Epidemiology of risk factors for age-related cataract. Surv Ophthalmol 39:323, 1995 137. Boukes RJ, van Balen AT, Bruynzeel DP: A retrospective study of ocular findings in patients treated with PUVA. Doc Ophthalmol 59:11, 1985 138. See JA, Weller P: Ocular complications of PUVA therapy. Australas J Dermatol 34:1, 1993 139. Geron RJ, MacDonald JS, Alberts AW, et al: On the etiology of subcapsular lenticular opacities produced in dogs receiving
HMG-CoA reductase inhibitors. Exp Eye Res 50:65–78, 1990 140. Hartman HA, Myers LA, Evans M, et al: The safety evaluation of fluvastatin, an HMG-CoA reductase inhibitor, in
beagle dogs and rhesus monkeys. Fundam Appl Toxicol 29:48–62, 1996 141. Rao PV, Robison WG Jr, Bettelheim F, et al: Role of small GTP-binding proteins in lovastatin-induced cataracts. Invest Ophthalmol Vis Sci 38:2313–2321, 1997 142. Boccuzzi SJ, Bocanegra TS, Walker JF, et al: No lens changes caused by simvastatin results from a prospective drug safety
study. Lens Eye Toxic Res 7:643–650, 1990 143. Lundh BL, Nilsson SE: Lens changes in matched normals and hyperlipidemic patients treated with
simvastatin for 2 years. Acta Ophthalmol (Copenh) 68:658–660, 1990 144. Schmidt J, Schmitt C, Hockwin O, et al: Ocular drug safety and HMG-CoA-reductase inhibitors. Ophthalmic Res 26:352–360, 1994 145. Harris ML, Bron AJ, Brown NA, et al: Absence of effect of simvastatin on the progression of lens opacities in
a randomised placebo controlled study. Oxford Cholesterol Study Group. Br J Ophthalmol 79:996–1002, 1995 146. Pedersen TR, Berg K, Cook TJ, et al: Safety and tolerability of cholesterol lowering with simvastatin during 5 years
in the Scandinavian Simvastatin Survival Study. Arch Intern Med 156:2085–2092, 1996 147. Qian W, Soderberg PG, Chen E, et al: 3 year simvastatin treatment and lens nuclear back scattering. Br J Ophthalmol 84:512–516, 2000 148. Schlienger RG, Haefeli WE, Jick H, Meier CR: Risk of cataract in patients treated with statins. Arch Intern Med 161:2021–206, 2001 149. Smeeth L, Hubbard R, Fletcher AE: Cataract and the use of statins: a case-control study. QJM 96:337–343, 2003 150. Boozalis GT, Purdue GF, Hunt JL, McCulley JP: Ocular changes from electrical burn injuries: a literature review and report
of cases. J Burn Care Rehabil 12:458, 1991 151. Noel LP, Clarke WN, Addison D: Ocular complications of lightning. J Pediatr Ophthalmol Strabismus 17:245, 1980 152. Duke-Elder S: System of ophthalmology. Vol. 11. St. Louis: CV Mosby, 1969:248 153. Jaeger EA: Cataract types. In: Tasman W, Jaeger EA (eds). Atlas of Clinical Ophthalmology. Philadelphia: Lippincott-Raven, 1995:6B |