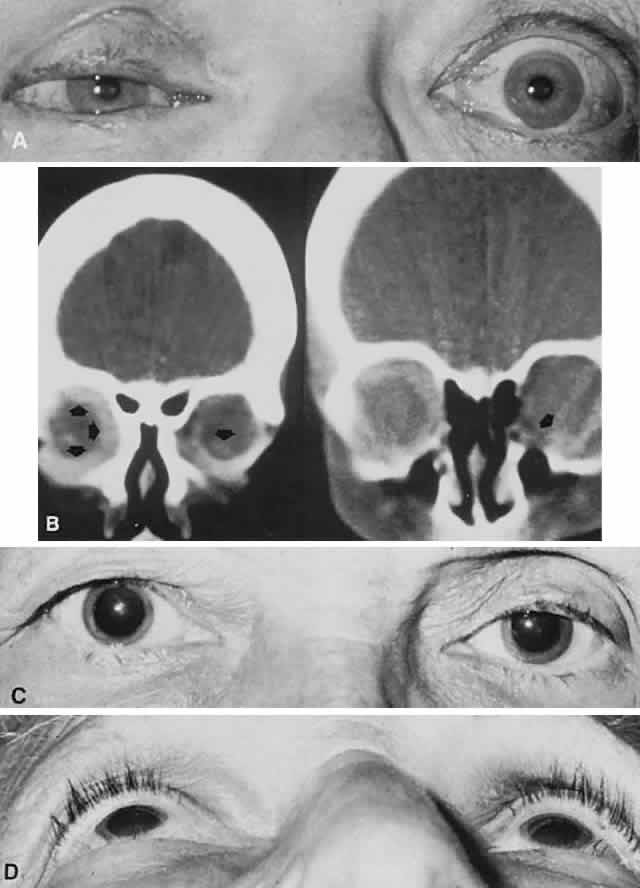
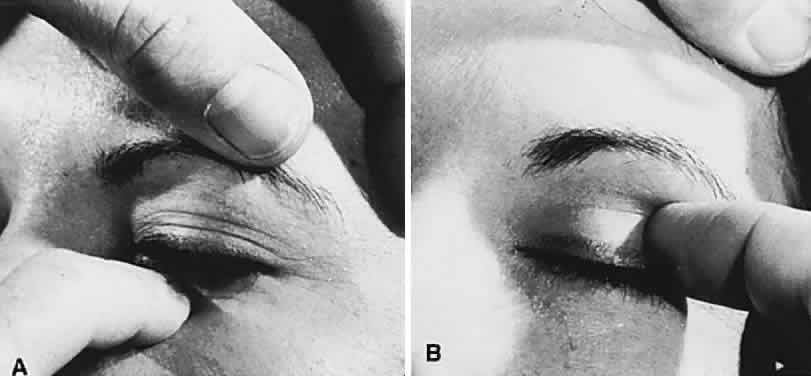
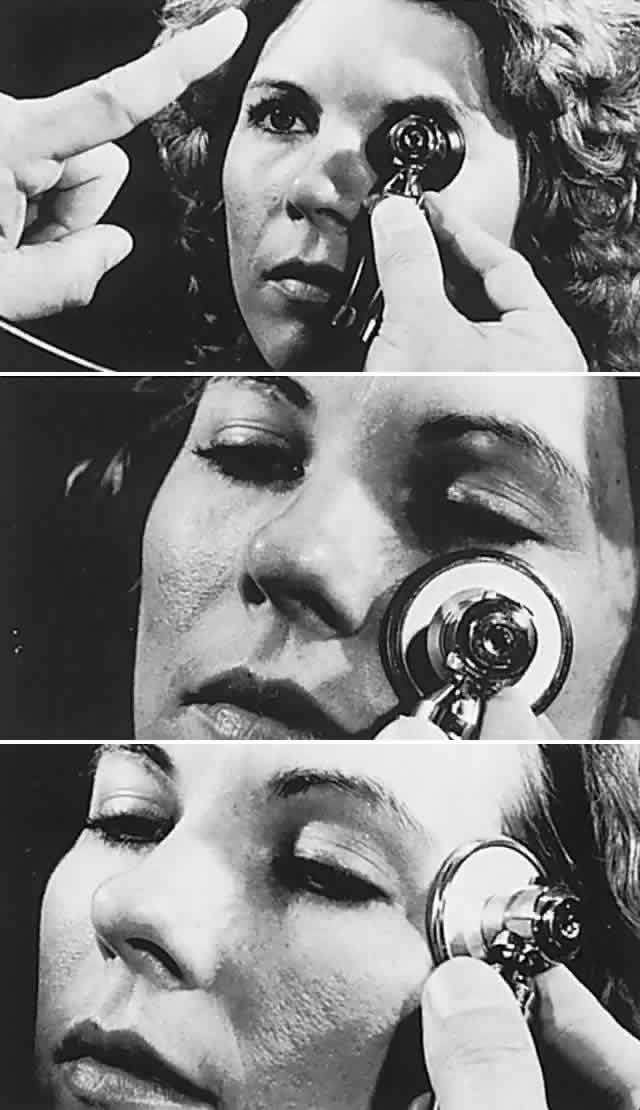
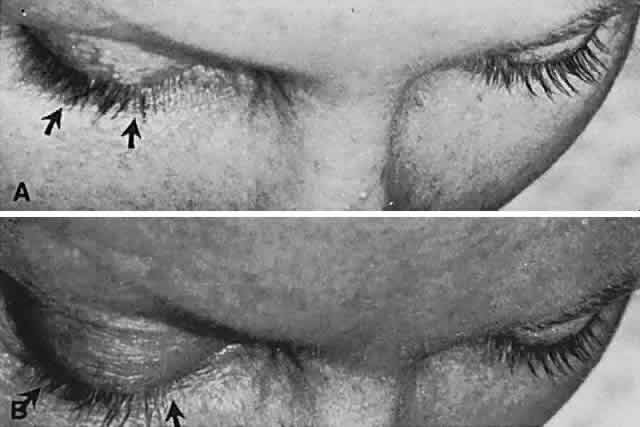
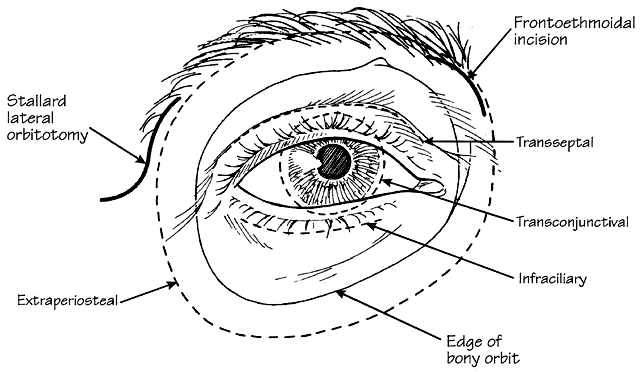
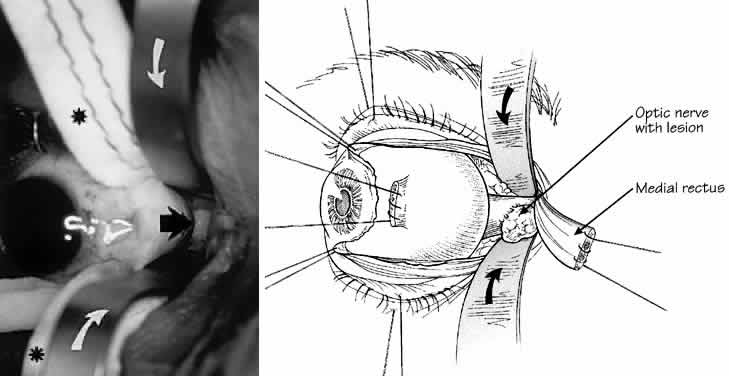
1. Rootman J: Diseases of the Orbit. Philadelphia, JB Lippincott, 1988 2. Char DH: Metastatic orbital tumors. Orbit 6:189, 1987 3. Katz SE, Rootman J, Goldberg RA: Secondary and metastatic tumors of the
orbit. In Tasman W, Jaeger EA (eds): Duane's Clinical Ophthalmology, Vol 2, Chap 46. Philadelphia, JB Lippincott, 1996 4. Jarrett WH, Gutman FA: Ocular complications of infection in the paranasal sinuses. Arch Ophthalmol 81:683, 1969 5. Hornblass A, Herschorn BJ, Stern K et al: Orbital abscess. Survey Ophthalmol 29:169, 1984 6. Mombaerts I, Goldschmeding R, Schlingeman RO et al: What is orbital pseudotumor? Survey Ophthalmol 41:66, 1996 7. Siatkowski RM, Capo H, Byrne SF et al: Clinical and echographic findings in idiopathic orbital myositis. Am J Ophthalmol 118:343, 1994 8. Rootman J, Hay E, Graeb D et al: Orbital-adnexal lymphangiomas. A spectrum of hemodynamically isolated vascular
hamartomas.Ophthalmology 93:1558, 1986 9. Katz B, Carmody R: Subperiosteal orbital hematoma induced by the Valsalva maneuver. Am J Ophthalmol 100:617, 1985. 10. Jacobson DM, Itani K, Digre KB et al: Maternal orbital hematoma associated with labor. Am J Ophthalmol 105: 547, 1998 11. Mannor GE, Rose GE, Moseley IF et al: Outcome of orbital myositis. Clinical features associated with recurrence.Ophthalmology 104:409, 1997 12. Manor RS, Yassur Y, Hoyt WF: Reading-evoked visual dimming. Am J Ophthalmol 121:212, 1996 13. Atta HR, Dick AD, Hamed LM et al: Venous stasis orbitopathy: a clinical and echographic study. Br J Ophthalmol 80:129, 1996 14. Gay AJ, Salmon ML, Windsor CE: Hering's law, the levators, and their relationship in disease states. Arch Ophthalmol 77:157, 1967 15. Migliori ME, Gladstone GJ: Determination of the normal range of exophthalmometric values for black
and white adults. Am J Ophthalmol 98:438, 1984 16. Musch DC, Frueh BR, Landis JR: The reliability of Hertel exophthalmometry. Observer variation between
physician and lay readers. Ophthalmology 92:1177, 1985 17. Cline RA, Rootman J: Enophthalmos: a clinical review. Ophthalmology 91:229, 1984 18. Miller MT, Spencer MA: Progressive hemifacial atrophy. A natural history study. Trans Am Ophthalmol Soc 93: 203, 1995 19. Soparkar CNS, Patrinely JR, Cuaycong MJ et al: The silent sinus syndrome: a cause of spontaneous enophthalmos. Ophthalmology 101:772, 1994 20. Bullock JD, Bartley GB: Dynamic proptosis. Am J Ophthalmol 102:104, 1986 21. Allen SJ, Naylor D: Pulsation of the eyeballs in tricuspid regurgitation. Can Med Assoc J 133:119, 1983 22. Stephens KF, Reinecke RD: Quantitative forced ductions. Trans Am Acad Ophthalmol Otolaryngol 71:324, 1967 23. Gamblin GT, Harper DG, Galentine P et al: Prevalence of increased intraocular pressure in Graves' disease. Evidence
of frequent subclinical ophthalmopathy.N Engl J Med 308:420, 1983 24. Ohtsuka K: Intraocular pressure and proptosis in 95 patients with Graves' ophthalmopathy. Am J Ophthalmol 124: 570, 1997 25. Reader AL: Normal variations of intraocular pressure on vertical gaze. Ophthalmology 89:1084, 1982 26. Spierer A, Eisenstein Z: The role of increased intraocular pressure on upgaze in the assessment
of Graves' ophthalmopathy. Ophthalmology 98:1491, 1991 27. De La Paz M, Boniuk M: Fundus manifestations of orbital disease and treatment of orbital disease. Surv Ophthalmol 40:3, 1995 28. Keltner JL: Is Graves' ophthalmopathy a preventable disease. Arch Ophthalmol 116:1106, 1998 29. Burch HB, Wartofsky L: Graves' ophthalmopathy: current concepts regarding pathogenesis and management. Endocr Rev 14:747, 1993 30. Tallstedt L, Lundell G, Torring O et al: Occurrence of ophthalmopathy after treatment for Graves' hyperthyroidism. N Engl J Med 326:1733, 1992 31. Bartalena L, Marcocci C, Bogazzi F et al: Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J Med 338:73, 1998 32. Sanders MD, Brown P: Acute presentation of thyroid ophthalmopathy. Trans Ophthalmol Soc UK 105:720, 1986 33. Liu GT, Heher KL, Katowitz JA et al: Prominent proptosis in childhood thyroid eye disease. Ophthalmology 103: 779, 1996 34. Trobe JD, Glaser JS, Laflamme P: Dysthyroid optic neuropathy. Clinical profile and rationale for management.Arch Ophthalmol 96:1199, 1978 35. Kazim M, Trokel S, Moore S: Treatment of acute Graves' orbitopathy. Ophthalmol 98:1443, 1991 36. Harnett AN, Doughty D, Hirst A, Plowman PN: Radiotherapy in benign orbital disease. II: Ophthalmic Graves' disease
and orbital histiocytosis X.Br J Ophthalmol 72:289, 1988 37. Kao SCS, Kendler DL, Nugent RA et al: Radiotherapy in the management of thyroid orbitopathy. Computed tomography
and clinical outcomes.Arch Ophthalmol 111:819, 1993 38. Bartalena L, Marcocci C, Bogazzi F et al: Relation between therapy for hyperthyroidism and the course of Graves' ophthalmopathy. N Engl J Med 338:73, 1998 39. Char D: Thyroid eye disease. Br J Ophthalmol 80:922, 1996 40. Bartley GB, Fatourechi V, Kadrmas EF et al: The treatment of Graves' ophthalmopathy in an incidence cohort. Am J Ophthalmol 121:200, 1996 41. Donahue SP, Schwartz G: Preseptal and orbital cellulitis in childhood. Ophthalmology 105:1902, 1998 42. Harril WC, Stewart MG, Lee AG et al: Chronic mucormycosis. Laryngoscope 106:1292, 1996. 43. De La Paz MA, Patrinely JR, Marines HM et al: Adjunctive hyperbaric oxygen in the treatment of bilateral cerebro-rhino-orbital
mucormycosis. Am J Ophthalmol 114:208, 1992 44. Klapper SR, Lee AG, Patrinely JR et al: Orbital involvement in allergic fungal sinusitis. Ophthalmology 104: 2094, 1997 45. Mombaerts I, Schlingemann RO, Goldschmeding R et al: Idiopathic granulomatous orbital inflammation. Ophthalmology 103:2135, 1996 46. Perry SR, Rootman J, White VA: The clinical and pathologic constellation of Wegener granulomatosis of
the orbit. Ophthalmology 104:683, 1997 47. Polito E, Leccisotti A: Prognosis of orbital lymphoid hyperplasia. Graefes Arch Clin Exp Ophthalmol 234:150, 1996 48. Jakobiec FA, Neri A, Knowles DM: Genotypic monoclonality in immunophenotypically polyclonal orbital lymphoid
tumors: a model of tumor progression in the lymphoid system. Ophthalmology 94:980, 1987 49. Logani S, Logani SC, Ali BH et al: Bilateral intraconal non-Hodgkin's lymphoma in a patient with acquired
immunodeficiency syndrome. Am J Ophthalmol 118:401, 1994 50. Reifler DM, Warzynski MJ, Blount WR et al: Orbital lymphoma associated with acquired immune deficiency syndrome (AIDS). Surv Ophthalmol 38:371, 1994 51. Peterson KL, Wang M, Canalis RF et al: Rhinocerebral mucormycosis: evolution of the disease and treatment options. Laryngoscope 107:855, 1997 52. Jakobiec FA, Nelson D: Lymphomatous, plasmacytic, and hematopoietic tumors
of the orbit. In Tasman W, Jaeger EA (eds): Duane's Clinical
Ophthalmology, Vol 2, Chap. 39. Philadelphia, JB Lippincott, 1993 53. Wright JE: Doyne lecture: current concepts in orbital disease. Eye 2:1, 1988 54. Katz SE, Rootman J, Vangveeravong S et al: Combined venous lymphatic malformations of the orbit (so-called lymphangiomas). Association
with noncontiguous intracranial vascular anomalies.Ophthalmology 105:176, 1998 55. Phelps CD, Thompson HS, Ossoinig KC: The diagnosis and prognosis of atypical carotid-cavernous fistula (red-eyed
shunt syndrome). Am J Ophthalmol 93:423, 1982 56. Borruat F-X, Bogousslavsky J, Uffer S et al: Orbital infarction syndrome. Ophthalmology 100:562, 1993 57. Galetta SL, Leahey A, Nichols CW et al: Orbital ischemia, ophthalmoparesis, and carotid dissection. J Clin Neuro-Ophthalmol 11:284, 1991 58. Erly WK, Carmody RF, Dryden RM: Orbital histiocytosis X. AJNR 16:1258, 1995 59. Kodsi SR, Shetlar DJ, Campbell RJ et al: A review of 340 orbital tumors in children during a 60-year period. Am J Ophthalmol 117:177, 1994 60. Kennerdell JS, Slamowitz T, Dekker A et al: Orbital fine needle aspiration biopsy. Am J Ophthalmol 99:547, 1985 61. Putterman AM: Management of blow-out fractures of the orbital floor: III. The conservative
approach.Surv Ophthalmol 35:292, 1991 62. Flanagan JC, McLachlan DL, Shannon GM: Orbital roof fractures. Neurologic and neurosurgical considerations.Ophthalmology 87:325, 1980 63. Iwamoto MA, Iliff NT: Management of orbital trauma. In Tasman W, Jaeger
EA, (eds): Duane's Clinical Ophthalmology, Vol 6, Chap. 135. Philadelphia, Lippincott, 1993 64. Meyer DR: Orbital fractures. In Tasman W, Jaeger EA, eds: Duane's
Clinical Ophthalmology, Vol 2, Chap. 48. Philadelphia, Lippincott, 1996 65. Char DH, Norman D: The use of computed tomography and ultrasonography in the evaluation of
orbital masses. Surv Ophthalmol 27:49, 1982 66. Moseley I: The contribution of X-ray computed tomography to the diagnosis and management
of orbital disease. Orbit 5:149, 1985 67. Johnson MH, DeFilipp GJ, Zimmerman RA, Savino PJ: Scleral inflammatory disease. AJNR 8:861, 1987 68. Ball JB: Direct oblique sagittal CT of orbital wall fractures. AJNR 8:147, 1987 69. Gilbard SM, Mahmood FM, Lagouros PA et al: Orbital blowout fractures. The prognostic significance of computed tomography.Ophthalmology 92:1523, 1985 70. Holt JE, O'Connor PS, Douglas JP et al: Extraocular muscle size comparison using standardized A-scan echography
and computerized tomography scan measurements. Ophthalmology 92:1351, 1985 71. Byrne SF, Glaser JS: Orbital tissue differentiation with standardized echography. Ophthalmology 90:1071, 1983 72. Jorgensen JS, Guthoff R: Differential diagnosis of the dilated superior ophthalmic vein by B-scan
ultrasonography. Orbit 5:259, 1986 73. Peyster RG, Savino PJ, Hoover ED, Schatz NJ: Differential diagnosis of the enlarged superior ophthalmic vein. J Comput Assist Tomogr 8:103, 1984 74. Carr WA, Baker RS, Lee C et al: NMR imaging of the orbit. An initial evaluation and comparison with CT. Orbit 6:85, 1987 75. Khanna RK, Pham CJ, Malik GM et al: Bilateral superior ophthalmic vein enlargement associated with diffuse
cerebral swelling. Report of 11 cases. J Neurosurg 86:893, 1997 76. Jane JA, Parks TS, Pobereskin LH et al: The supraorbital approach: technical note. Neurosurgery 11:537, 1982 77. Prummel MF, Mourits MP, Berghout A et al: Prednisone and cyclosporine in the treatment of severe Graves' ophthalmopathy. N Engl J Med 321:1353, 1989 78. Hurwitz JJ, Birt D: An individualized approach to orbital decompression in Graves' orbitopathy. Arch Ophthalmol 96:1199, 1978 79. Mourits M, Koornneef L, Wiersinga WM et al: Orbital decompression for Graves' ophthalmopathy by inferomedial, by inferomedial
plus lateral, and by coronal approach. Ophthalmology 97:636, 1990 80. Lyons CJ, Rootman J: Orbital decompression for disfiguring exophthalmos in thyroid orbitopathy. Ophthalmology 101:223, 1994 81. Harting F, Koornneef L, Peeters HJ et al: Decompression surgery in Graves' orbitopathy—a review of 14 years' experience
at the orbita centrum, Amsterdam. Dev Ophthalmol 20:185, 1989 82. Garrity JA, Fatourechi V, Bergstralh EJ et al: Results of transantral orbital decompression in 428 patients with severe
Graves' ophthalmopathy. Am J Ophthalmol 116: 533, 1993 83. Kennedy DW, Goodstein ML, Miller NR et al: Endoscopic transnasal orbital decompression. Arch Otolaryngol Head Neck Surg 116:275, 1990 84. Leone CR, Piest KL, Newman RJ: Medial and lateral wall decompression for thyroid ophthalmopathy. Am J Ophthalmol 108:160, 1989 85. Deleted in proof 86. Carter KD, Frueh BR, Hessburg TP et al: Long term efficacy of orbital decompression for compressive optic neuropathy
of Graves' eye disease. Ophthalmology 98:1435, 1991 87. Olivari N: Transpalpebral decompression of endocrine ophthalmopathy by removal of
intraorbital fat: experience with 147 operations over 5 years. Plast Reconstr Surg 187:627, 1991 88. Warren JD, Spector JG, Burde R: Long term follow up and recent observations on 305 cases of orbital decompression
for dysthyroid orbitopathy. Laryngoscope 99:35, 1989 89. Harvey JT: Orbital decompression for Graves' disease leaving the periosteum intact. Ophthalmol Plast Reconstr Surg 5:199, 1989 90. Pitz GK, Muller-Forell W, Hommel G: Randomized trial of intravenous immunoglobulins versus prednisolone in
Graves' ophthalmopathy. Clin Exp Immunol 106:197, 1996 91. DeWecker L: On incision of the optic nerve in cases of neuroretinitis. Rep Int Ophthalmol Congr 4:11, 1872 92. Tse DT, Nerad JA, Anderson RL et al: Optic nerve sheath fenestration in pseudotumor cerebri: a lateral orbitotomy
approach. Arch Ophthalmol 106:1458, 1988 93. Kersten RC, Kulwin DR: Optic nerve sheath fenestration through a lateral canthotomy incision. Arch Ophthalmol 111:870, 1993 94. Corbett JJ, Nerad JA, Tse DT et al: Results of optic nerve sheath fenestration for pseudotumor cerebri: the
lateral orbitotomy approach. Arch Ophthalmol 106:1391, 1988 95. Brourman ND, Spoor TC, Ramochi JM: Optic nerve sheath decompression provides long-term visual improvement
for pseudotumor cerebri. Arch Ophthalmol 106:1378, 1988 96. Steinsapir KD, Goldberg RA: Traumatic optic neuropathy. Surv Ophthalmol 38:487, 1994 97. Bilyk JR, Joseph MP: Traumatic optic neuropathy. Semin Ophthalmol 9:200, 1994 98. Joseph MP: Traumatic optic neuropathy. Ophthalmol Clin North Am 8:693, 1995 99. Anderson RL, Panje WR, Gross CE: Optic nerve blindness following blunt trauma. Ophthalmology 89:445, 1982 100. Aitken PA, Sofferman RA: Traumatic optic neuropathy. Ophthalmol Clin North Am 4:479, 1991 101. Crompton MR: Visual lesions in closed head injury. Brain 93:785, 1970 102. Manfredi SJ, Raji MR, Sprinkle PM: Computerized tomographic scan findings in facial fractures associated with
blindness. Plast Reconstr Surg 68:479, 1981 103. Miller NR. The management of traumatic optic neuropathy. Arch Ophthalmol 108:1086, 1990 104. Spoor TC, Hartel WC, Lensink DB et al: Treatment of traumatic optic neuropathy with corticosteroids. Am J Ophthalmol 110:665, 1990 105. Bracken MB, Shepard MJ, Collins WF et al: A randomized, controlled trial of methylprednisolone or naloxone in the
treatment of acute spinal-cord injury results of the Second National
Acute Spinal Cord Injury Study. N Engl J Med 322:1405, 1990 106. Call NB. Decompression of the optic nerve in the optic canal. Ophthalmic
Plast Reconstr Surg 2:133, 1996 107. Takahashi M, Itoh M, Kaneko M, et al: Microscopic intranasal decompression of the optic nerve. Arch Otorhinolaryngol 246:113, 1989 108. Amrith S, Pham T, Chee C et al: Visual recovery following transethmoidal optic nerve decompression in traumatic
optic neuropathy. Ophthalmic Surg 24:49, 1993 109. Knox BE, Gates GA, Berry SM. Optic nerve decompression via the lateral
facial approach. Laryngoscope 100:458, 1990 110. Joseph MP, Lessell S, Rizzo J et al: Extracranial optic nerve decompression for traumatic optic neuropathy. Arch Ophthalmol 108:1091, 1990 111. Levin LA, Joseph MP, Rizzo JF et al: Optic canal decompression in indirect optic nerve trauma. Ophthalmology 101:566, 1994 112. Sofferman RA: Sphenoethmoidal approach to the optic nerve. Laryngoscope 91:184, 1981 113. Cook MW, Levin LA, Joseph MP et al: Traumatic optic neuropathy. Arch Otolaryngol Head Neck Surg 122:389, 1996 |