ORBITAL CELLULITIS
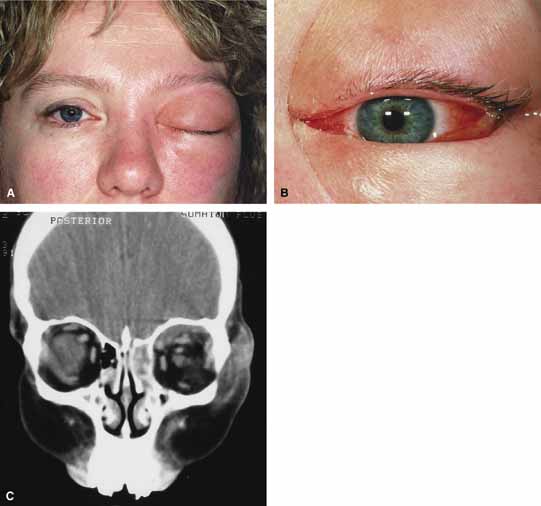
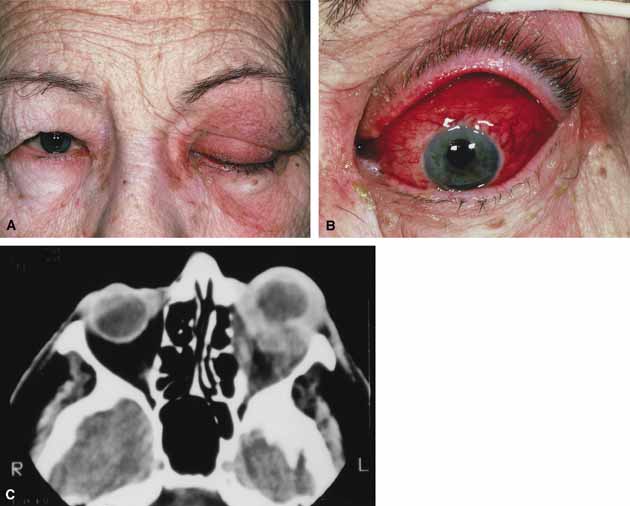
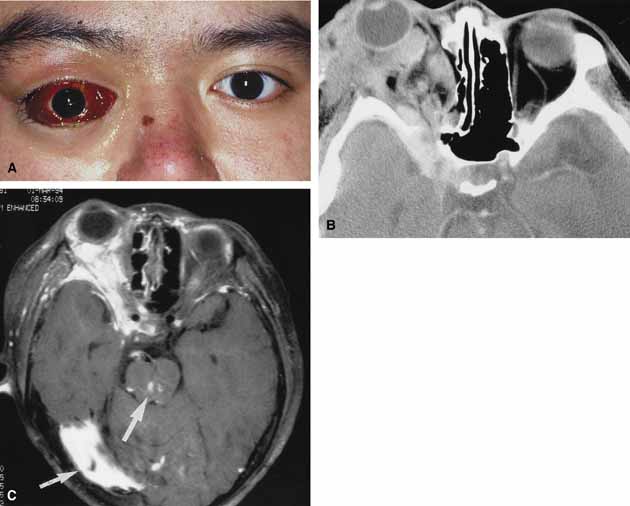
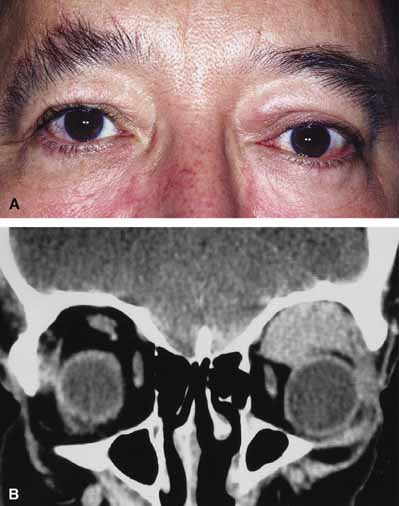
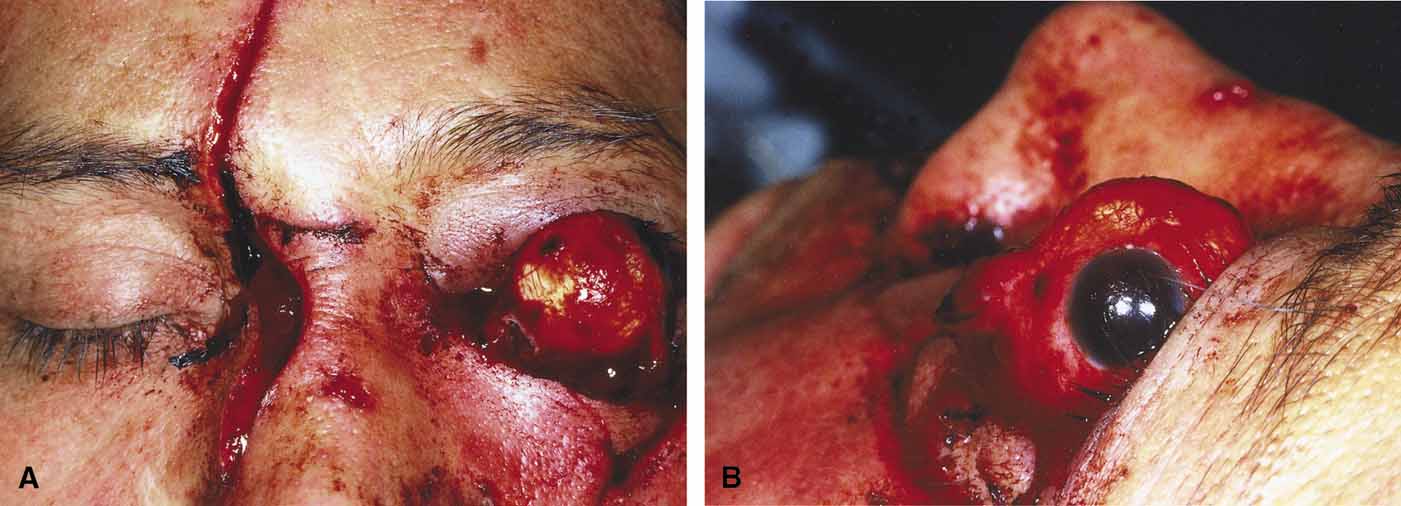
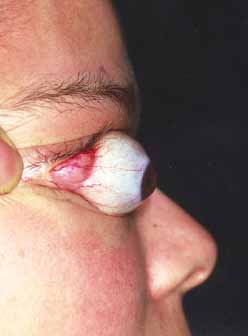
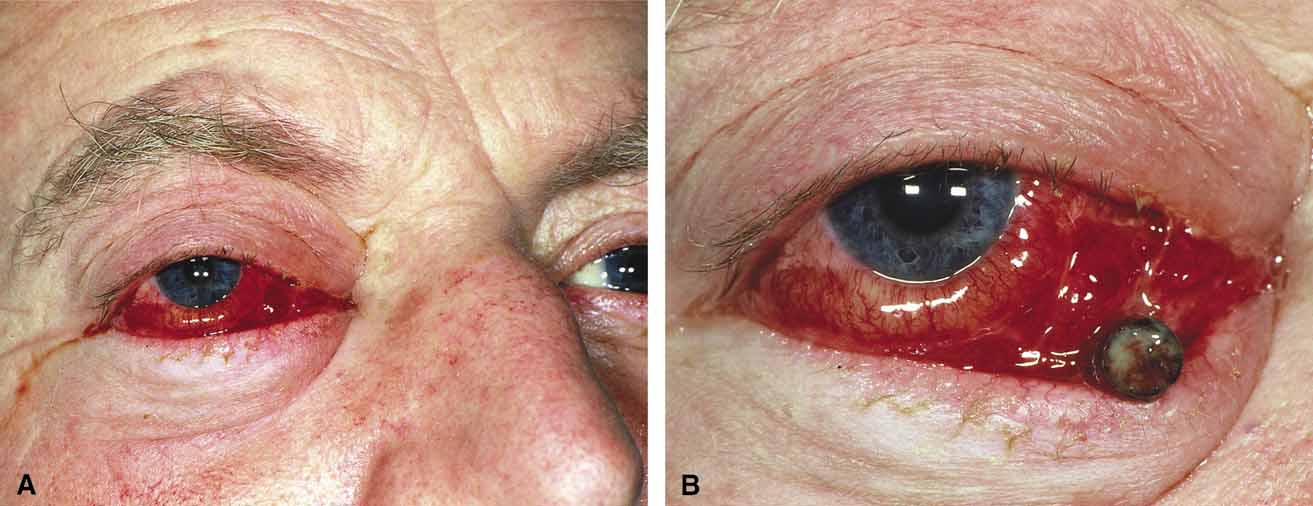
Orbital cellulitis is the model for acute inflammation and a major cause of orbital inflammation in adults.2 It is characterized by a rapid development (over 1 to 2 days) of inflammatory signs and symptoms. These include: eyelid swelling, redness, warmth, pain, conjunctival injection, chemosis, proptosis, and mobility impairment with or without reduced vision. These patients generally feel unwell (malaise) and are febrile (Fig. 1A and 1B). The malaise and fever are key features in differentiating cellulitis from a rapid-onset nonspecific orbital inflammation (pseudotumor) and should be obtained from the patient's history.
The majority of orbital cellulitis is secondary to extension from an adjacent sinus infection (Fig. 1C). Organisms gain access to the orbit directly through the thin ethmoidal bone, through congenital or acquired dehiscences in the thin orbital walls, pass through preexisting orbital foramina, pass retrogradely through the valveless venous orbital system, or along the veins as a periphlebitis. Orbital cellulitis may also be secondary to an endophthalmitis, systemic bacteremia (e.g., after dental work), infection of a nearby skin wound, dacryocystitis, or penetrating trauma.
The history and physical examination are crucial in distinguishing between preseptal and true orbital cellulitis. The orbital septum delineates the anterior eyelid soft tissue from the orbital soft tissue. Infections anterior to the septum are classified as preseptal cellulitis while those posterior to the septum are termed orbital cellulitis. Recognition of true orbital involvement is important not only because of the threatened visual loss associated with the orbital involvement but also because of the potential for central nervous system complications including cavernous sinus thrombosis, meningitis, and death.
Preseptal cellulitis is characterized by lid edema, erythma, and discomfort. Inflammation posterior to the orbital septum (orbital cellulitis) is heralded by the development of pain, chemosis, proptosis, motility disturbance, and visual deterioration. The extent of ocular involvement in preseptal and orbital cellulitis can be determined by assessing visual acuity, extraocular motility, pupillary reaction, color vision, confrontation visual fields, intraocular pressure, and optic nerve head appearance. Evidence of facial/head trauma or surgical wounds may be apparent. Constitutional signs such as fever, lethargy, and irritability should be sought. The examiner should assess for meningeal signs and neurologic defects. After the initial examination, the physician should follow patients with orbital cellulitis with at least daily assessments of visual acuity, motility, and pupil reaction.
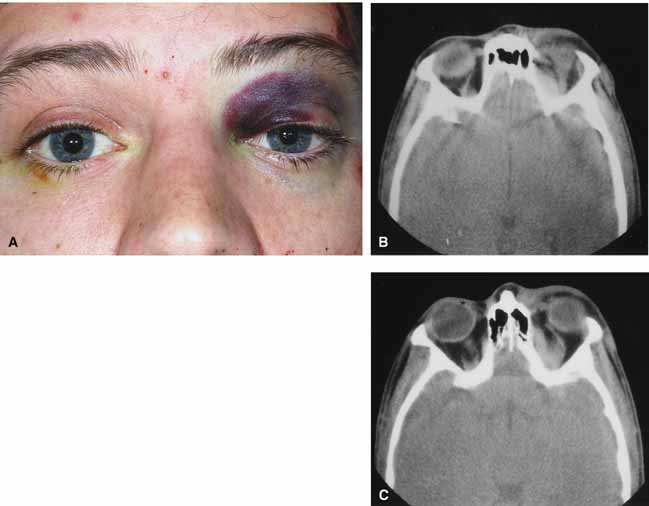
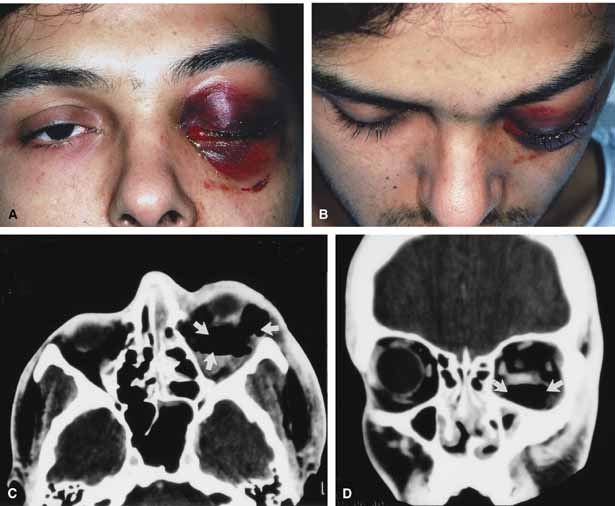
Progression of the disease process despite appropriate antibiotic therapy suggests abscess formation. Orbital abscesses may be either localized, diffuse, or subperiosteal (Fig. 1C). Subperiosteal abscesses most commonly occur along the medial wall and may expand rapidly, compromising optic nerve function even in the absence of many signs of infection.3–6 Several mechanisms may contribute to visual deterioration including direct optic nerve compression, elevation of the intraorbital pressure, and proptosis causing a “stretch” optic neuropathy. Clinically, the eye typically is displaced away from the subperiosteal abscess, and orbital imaging shows a convex mass adjacent to the involved sinus.
Orbital infection with potential orbital abscess formation occurs when bacteria break through the periorbita and gain access to the extraoconal or intraconal space. Diffuse or localized orbital abscess formation may also lead to visual loss through an increase in intraorbital pressure, posterior ischemic optic neuropathy, optic nerve inflammation, or vasculitis. Acute visual loss in the presence of an orbital infection is almost always a surgical emergency in which immediate drainage of the abscess is required to save vision. Continued posterior extension of the infection may result in an orbital apex syndrome, decreased function of cranial nerves,2–6 thrombosis of the cavernous sinus, and even death. Hallmarks of cavernous sinus thrombosis include cranial neuropathy and central neurologic impairment.7
Organisms responsible for orbital cellulitis vary widely and may include Staphylococcous aureus, Streptococcus species, as well as a mixture of aerobes and anaerobic organisms. Recent studies indicate that streptococcus is the most common cause of orbital cellulitis associated with sinusitis in children.8 With increasing age the pathogens increase in complexity. In patients older than 15 with subperiosteal abscesses, polymicrobial infections are typical with anaerobes cultured in every case.5
Orbital imaging in the axial and coronal plane should be obtained in all patients suspected of having orbital cellulitis. Computed tomography (CT) is preferred to magnetic resonance imaging (MRI) because the orbital tissues have higher contrast and bone is well visualized. Axial CT views allow evaluation of the medial orbit and ethmoid sinuses, whereas coronal scans image the orbital roof, floor, frontal, and maxillary sinus. A subperiosteal abscess appears as a homogeneous opacification between the orbital wall and the displaced periorbita.6 Contrast agents are not necessary to visualize a subperiosteal abscess.
Management of orbital cellulitis is dictated by the rapidity of onset. Oral antibiotics may be appropriate for mild cases whereas intravenous antibiotics are required for more fulminant cases. Antibiotic coverage should be broad spectrum and include coverage for gram-positive organisms and anaerobes. Examples include cloxicillan and clindamycin or a late-generation cephalosporin and clindamycin. In those patients allergic to penicillin agents, erythromycin and clindamycin or vancomycin and clindamycin are considerations.
Most patients with orbital cellulitis also benefit from a nasal decongestant as well as an oral decongestant and warm compresses to the affected site (10 minutes out of every hour). Daily or more frequent reexaminations are required depending on the fulminancy of the disease. Once antibiotics are initiated, a 24-hour wait-and-watch period is generally observed (unless the process is aggressive and rapidly developing). If there is no progression at 24 hours and the vision is stable, continued observation is appropriate. If the orbital cellulitis is rapidly developing and fulminant, frequent assessments (every 2 to 4 hours) are required. If the vision, motility, or neurologic status is deteriorating, immediate surgical intervention may be required.
The indication for surgery in a patient with orbital cellulitis has been controversial over the years. The simple presence of a subperiosteal abscess (SPA) was at one time an indication to drain but this is no longer always the case. Providing the vision is normal, the SPA may resolve with medical management. The clinical course, and not the radiographic appearance should dictate management.5,6,9 Surgical therapy may be influenced by many factors including the visual status, size, and location of the SPA; intracranial complications; the sinus involved; the presumed pathogenesis, and the anticipated bacterial response to antibiotic treatment.10 Immediate drainage of SPAs and sinuses is recommended for patients of any age whose vision is compromised. Urgent drainage (as soon as practical) should be considered for large SPAs, extensive superior or inferior abscesses, intracranial complications at the time of presentation, frontal sinusitis where the risk of intracranial extension is increased, and in those suspected of having anaerobes (postdental procedures). However, cookbook approaches should never take the place of good clinical judgement and therapeutic decisions regarding early drainage versus medical management with observation are ultimately up to the managing physician.10 Older children (older than 9 years of age) and adults benefit from early surgical drainage. Although surgical treatment does not guarantee rapid resolution, a complicated course is more likely without it.10
Abscesses may also develop within the intraconal space. The prescence of an abscess within the orbital tissue coupled with any signs of visual loss, afferent pupillary defect, or a significant motility defect should prompt emergent surgery. If visual acuity is stable and extraocular motility essentially full, initial treatment may consist of empiric antibiotic therapy and close observation with serial CT scans.
The prescence of neurologic symptoms such as altered mental status or seizures implies intracranial extension with potential intracranial abscess. Infection may spread from the sinuses to the intracranial cavity via retrograde thrombophlebitis, directly through osteitic bone or from congenital or acquired bony defects. In the past, intracranial abscess formation had a poor prognosis with a high mortality rate. Successful management of suppurative abscesses requires early recognition of the disease process, intravenous antibiotics, serial neuroimaging, and surgical management of at least the orbit and sinus disease and often the intracranial disease.11
PHYCOMYCOSIS (RHINO-ORBITAL MUCORMYCOSIS AND ORBITAL ASPERGILLOSIS)
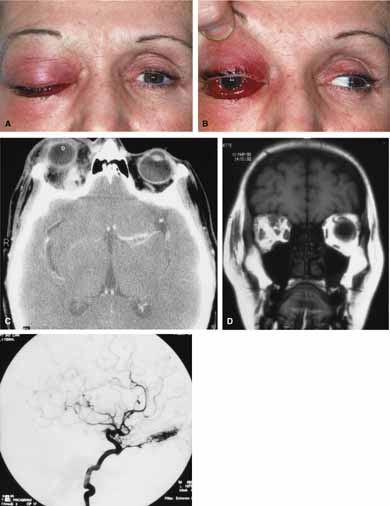
Rhino-orbital mucormycosis, a fungal infection of class Phycomycetes and order mucorales, is notable for its high morbidity and mortality.12,13 Orbital involvement is an acute, aggressive, and often lethal infection if not recognized early. Phycomycetes (common bread molds) are ubiquitous fungi occurring in soil, air, skin, body orifices, manure, and food including fruit.13 Inoculation occurs by inhalation reaching the nasopharynx and oropharynx. At this stage most patients are able to contain the disease. However, individuals whose cellular and humoral defense mechanisms have been compromised by disease or immunosuppressive treatment may not be able to generate an adequate response. The fungus may then spread to the paranasal sinus, orbit, meninges, and brain by direct extension.13 Mucormycosis preferentially involves blood vessel walls resulting in vascular occlusion, thrombosis, and infarction.14 This frequently affects the ophthalmic artery and in more serious cases may involve the internal carotid artery and cavernous sinus.
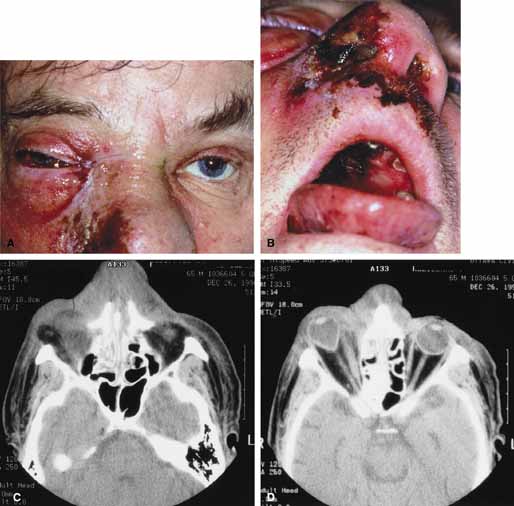
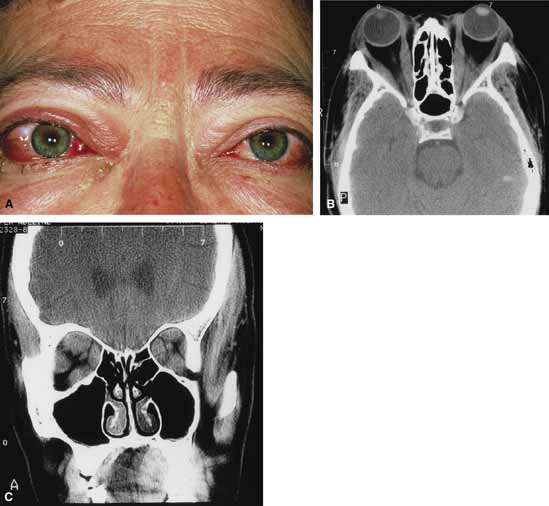
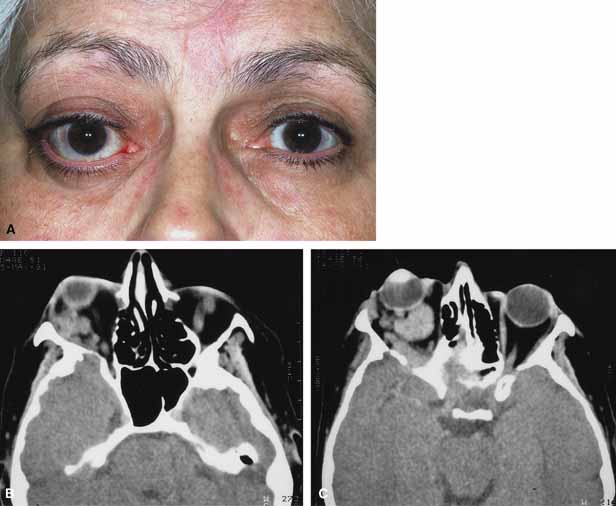
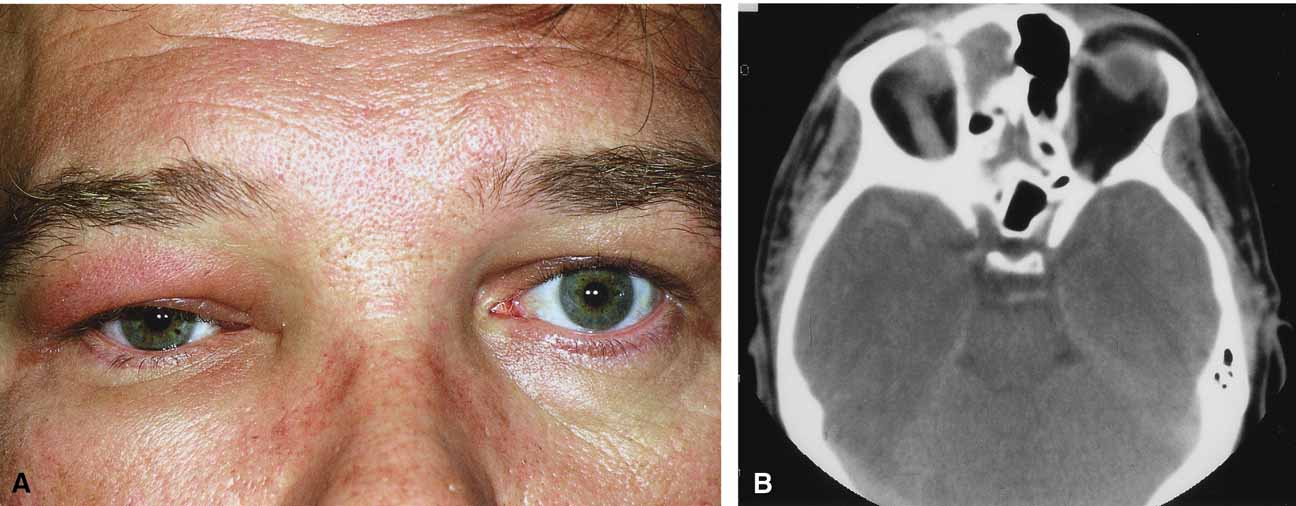
Although there have been a few reports of mucormycosis occurring in healthy individuals, virtually all other patients have had previous severe underlying disease. The patient most vulnerable to this infection is an one with uncontrolled diabetes with ketoacidosis. A host of other conditions also predispose patients to the disease including: multiple myeloma, lymphoma, organ transplantation with immunosuppresion, chemotherapy, corticosteroid treatment, acquired immunodeficiency syndrome, etc.(Fig. 2A).12–14 Mortality is extremely high for patients with phycomycosis infection, and successful treatment is contingent on early recognition and prompt treatment.
A characteristic pattern of clinical symptoms and signs occurs, the recognition of which should lead to the immediate institution of antifungal treatment and possible surgical intervention to increase the patient's chances of survival. Early diagnosis while the disease is still somewhat anatomically confined is essential for a more favorable outcome.12–14
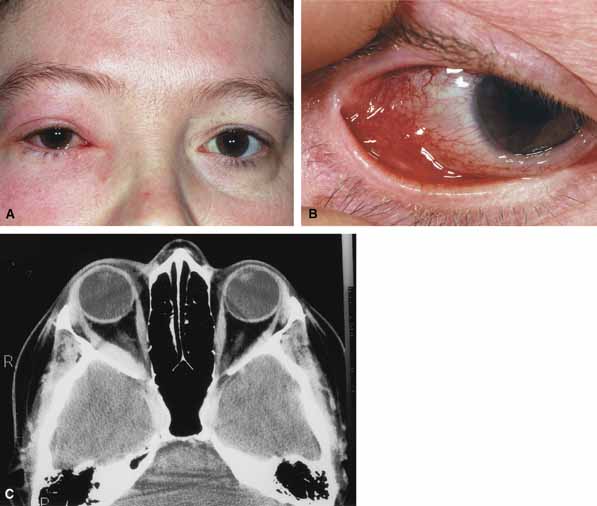
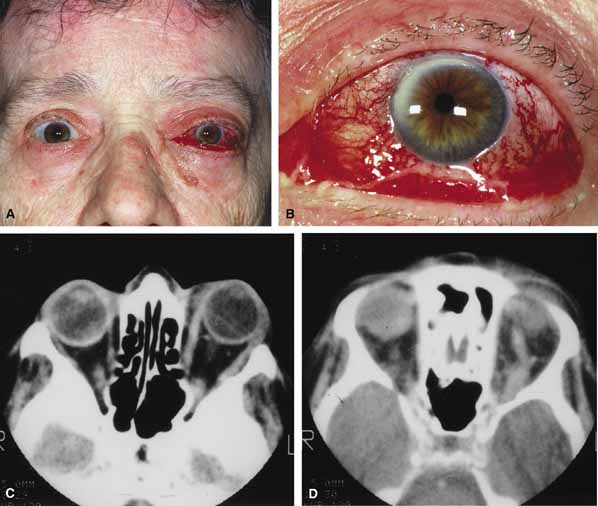
Characteristic features of orbital mucormycosis include an immunocompromised patient with sinusitis, pharyngitis or nasal discharge who develops cellulitis of the face or lid. Signs and symptoms include orbital/periorbital pain, acute proptosis, abrupt visual loss, orbital apex syndrome with acute motility changes (external ophthalmoplegia), pupillary changes (internal ophthalmoplegia), ptosis, and decreased corneal sensation. Infarction of tissue results in black eschar formation of the skin, nasal mucosa and hard palate (Fig. 2A and 2B).12,13 With intracranial extension, the patient generally become obtunded, develops convulsions, contralateral hemianaesthesia or hemiplegia, and lapses into coma.
CT scanning demonstrates an orbital mass often with bone destruction and sinus involvement (Fig. 2C and 2D). The diagnosis is confirmed by biopsy of involved tissue with demonstration of characteristic nonseptate, large, branching hyphae, which can be seen on routine hematoxylin and eosin stains. Material should be submitted for both frozen and conventional paraffin-embedded sections. Frozen sections are not always definitive and the surgeon must have considerable confidence in the skill of the pathologist. 13 Management includes: (1) early definitive diagnosis; (2) correction of any underlying metabolic disturbance; (3) wide local excision with debridement of all involved and devitalized oral, nasal, sinus and orbital tissue; (4) establishment of adequate sinus and orbital drainage; (5) daily irrigation and packing of the involved orbital and paranasal areas with amphotericin B; and (6) intravenous amphotericin B.12,13,15
The extent of surgical excision should balance the degree of morbidity and mutilation against the life-threatening risk this organism represents. In limited cases, surgical excision may be confined to those tissues clearly infarcted. Should infection be extensive as demonstrated by widespread necrosis, then aggressive surgery, including exenteration of the orbit and any involved paranasal sinuses, may prove necessary and lifesaving.13
ORBITAL ASPERGILLOSIS
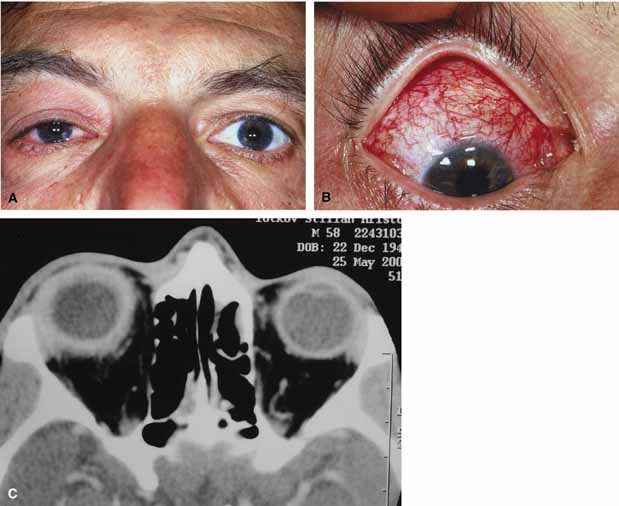
Aspergillus, a fungus of the Ascomycetes class, is a common environmental organism that may colonize the aerodigestive tract. Although widespread, the fungus has a low intrinsic virulence and clinically apparent aspergillosis is rare in the immunocompetant host. Invasive aspergillosis, similar to mucormycosis, occurs more often in the immunocompromised host, including patients undergoing transplantation or steroid therapy, neutropenic patients undergoing chemotherapy, alcoholics, patients with diabetes, and patients with acquired immune deficiency syndrome (AIDS).16–20 Orbital involvement may be slowly progressive or abrupt in onset with orbital inflammation, proptosis, pain, ophthalmoplegia, and sudden loss of vision.16,19 Fulminant aspergillus infection of the nose, paranasal sinus, and orbit often associated with intracranial extension has been reported with increasing frequency in immunocompromised individuals.17,18 In these patients, local invasion of the sinus mucoperiosteum produces a rapidly progressive gangrenous necrosis.18 With bone destruction, infection may extend into the orbit and intracranial cavity; the latter has a poor prognosis. The fungus may also spread by hematogenous routes.17 Imaging studies reveal sinus involvement, heterogenous soft tissue masses with bony erosion, and calcification.16 A definitive diagnosis of aspergillosis is based on tissue biopsy and fungal cultures.
Treatment of invasive sino-orbital aspergillosis involves aggressive surgical debridement combined with a systemic antifungal agent. Intravenous amphoticin B has been the mainstay of medical therapy but toxic side effects, especially renal, require discontinuing the medication in some patients. Newer systemic antifungal agents include liposomal amphotericin B (fewer renal toxic effects) and oral intraconazole.21–24 Adjuvant local irrigation of amphotencin B has also been recommended.24 For patients unable or unwilling to undergo surgery, intralesional injection of amphotericin B has been used successfully as palliative treatment.25 Despite aggressive therapy, the mortality in those with invasive sino-orbital aspergillosis remains high.17