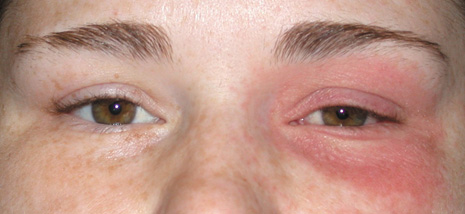
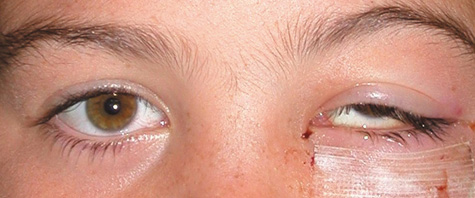
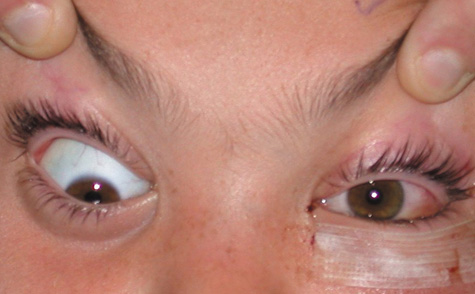
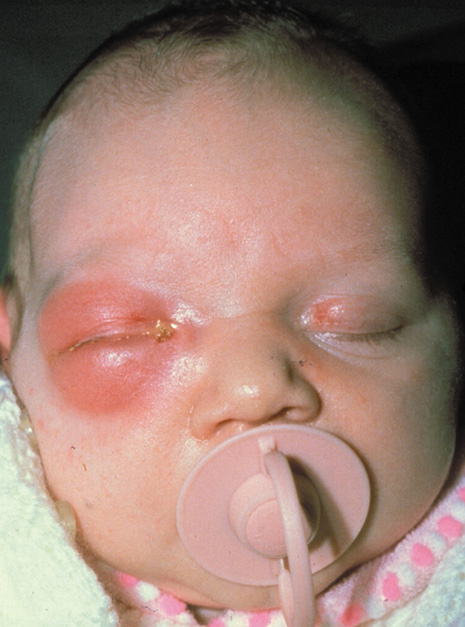
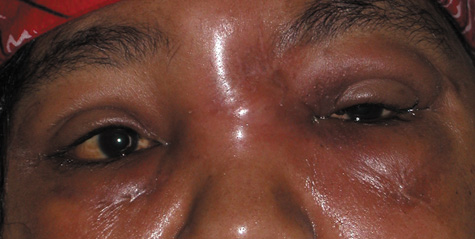
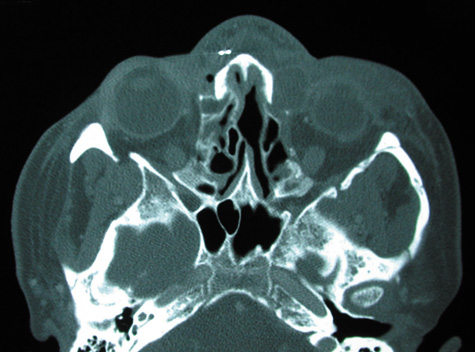
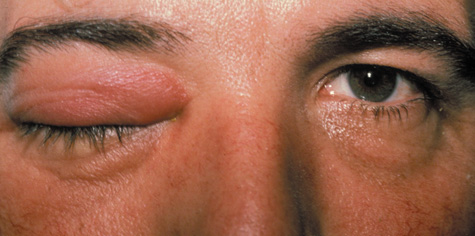
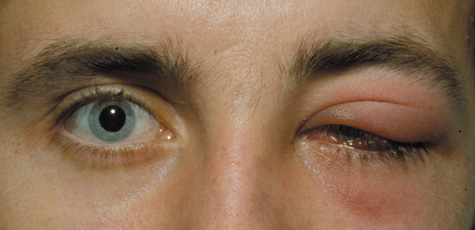
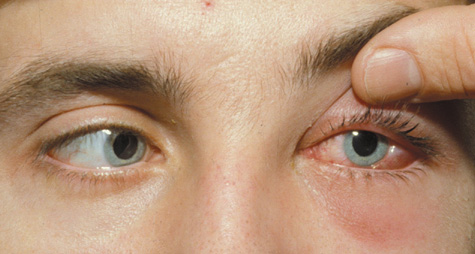
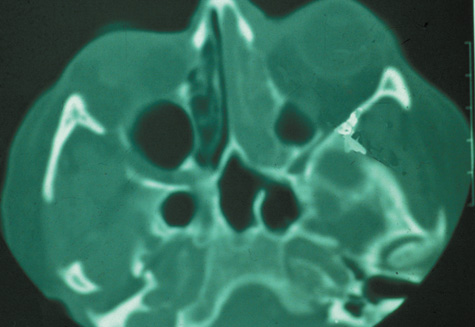
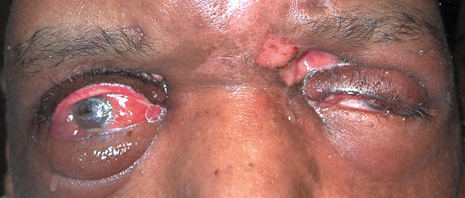
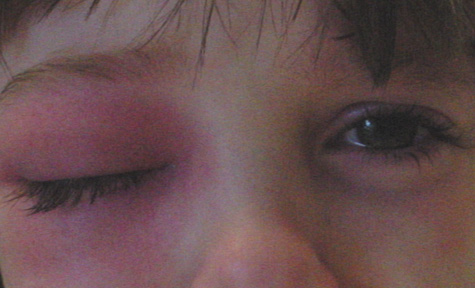
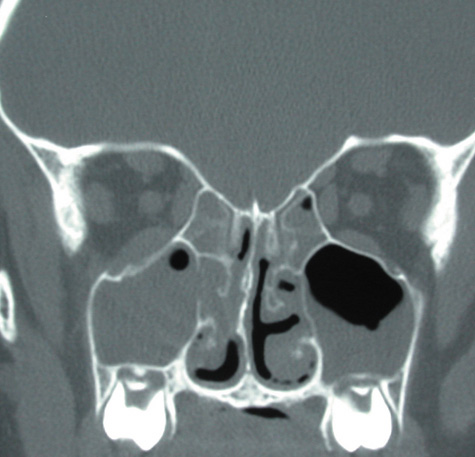
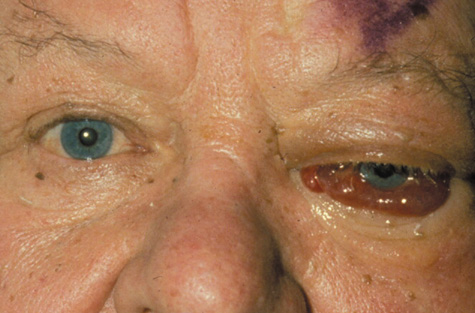
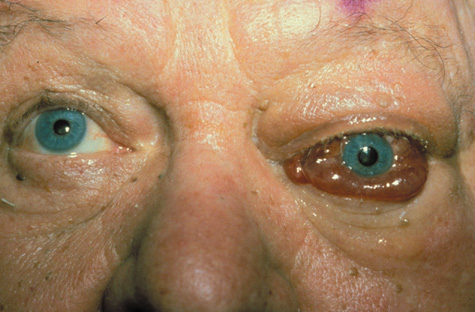
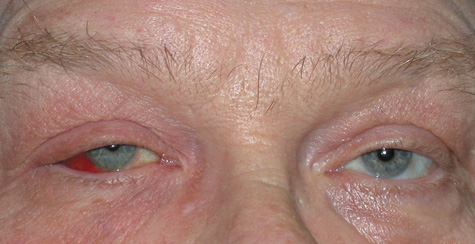
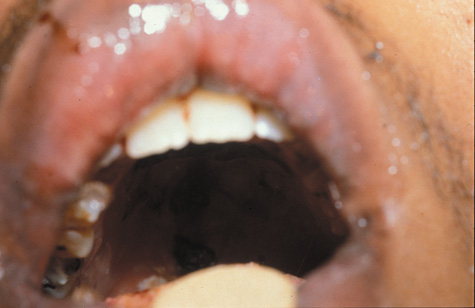
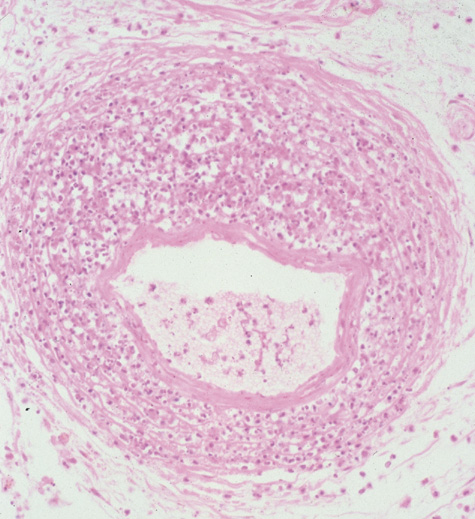
1. Jackson K, Baker SR: Clinical implications of orbital cellulitis. Laryngoscope 96:568, 1986 2. Watters EC, Wallar H, Hiles DA, Michaels RH: Acute orbital cellulitis. Arch Ophthalmol 94:785, 1976 3. Hawkins DB, Clark RW: Orbital involvement in acute sinusitis. Clin Pediatr 16:464, 1977 4. Gellady AM, Shulman ST, Ayoub EM: Periorbital and orbital cellulitis in children. Pediatrics 61:272, 1978 5. Rubinstein JB, Handler SD: Orbital and periorbital cellulitis in children. Head Neck Surg 5:15, 1982 6. Israele V, Nelson JD: Periorbital and orbital cellulitis. Pediatr Infect Dis J 6:404, 1987 7. Shapiro ED, Wald ER, Broznski BA: Periorbital cellulitis and paranasal sinusitis: A reappraisal. Pediatr Infect Dis J 1:91, 1982 8. Schramm VL, Curtin HD, Kennerdell JS: Evaluation of orbital cellulitis and results of treatment. Laryngoscope 92:732, 1982 9. Schramm VL, Myers EN, Kennerdell JS: Orbital complications of acute sinusitis: Evaluation, management, and outcome. Trans Am Acad Otolaryngol 86:221, 1978 10. Haynes R, Cramblett H: Acute ethmoiditis. Am J Dis Child 114:261, 1967 11. Gamble RE: Acute inflammations of the orbit in children. Arch Ophthalmol 10:483, 1933 12. Weiss A, Friendly D, Eglin K, et al: Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology 90:195, 1983 13. Krohel GB, Krauss HR, Christensen RE, Minckler D: Orbital abscess. Arch Ophthalmol 98:274, 1980 14. Ambati BK, Ambati J, Azar N, Stratton L, Schmidt EV: Periorbital and orbital cellulitis before and after the advent of haemophilus
influenzae type B vaccination. Ophthalmology 107:1450, 2000 15. Powell KR: Orbital and periorbital cellulitis. Pediatr Rev 16:163, 1995 16. Donahue Sp, Khoury JM, Kowalski RP: Common ocular infections. Drugs 52:526, 1996 17. Gans H, Sekula J, Wlodyka J: Treatment of acute orbital complications. Arch Ophthalmol 100:329, 1974 18. Jain A, Rubin PAD: Orbital cellulitis in children. Int Ophthalmol Clin 41:71, 2001 19. Rumelt S, Rubin PAD. Potential sources of orbital cellulitis. Int Ophthalmol Clin 36:207, 1996 20. Molarte AB, Isenberg SJ: Periorbital cellulits in infancy. J Pediatr Ophthalmol Strabismus 26:232, 1989 21. Williamson-Nobel FA: Diseases of the orbit and its contents secondary to pathological conditions
of the nose and paranasal sinuses. Ann R Coll Surg 15:46, 1954 22. Wulc AE, Adams JL, Dryden RM: Cerebrospinal fluid leakage complicating orbital exenteration. Arch Ophthalmol 107:827, 1989 23. Whitnall SE: The Anatomy of the Human Orbit and Accessory Organ of Vision. 2nd ed, pp 29–35.New York: Oxford University Press, 1932 24. Harris GJ: Subperiosteal abscess of the orbit. Arch Ophthalmol 101:751, 1983 25. Krohel GB, Kraus HR, Winnick J: Orbital abscesses: presentation, diagnosis, therapy, and sequelae. Ophthalmology 89:492, 1982 26. Williams BJ, Harrison HC: Subperiosteal abscesses of the orbit due to sinusitis in childhood. Aust NZ J Ophthalmol 19:29, 1991 27. Stammberger H: Functional Endoscopic Sinus Surgery. p 68. Philadelphia: BC Decker, 1991 28. Batson OV: Relationship of the eye to the paranasal sinuses. Arch Ophthalmol 16:322, 1936 29. Chandler JR, Langenbrunner DJ, Stevens ER: The pathogenesis of orbital complications in acute sinusitis. Laryngoscope 80:1414, 1970 30. Hubert L: Orbital infection due to nasal sinusitis. NY State J Med 37:1559, 1937 31. Vail DT: Orbital complications in sinus disease: a review. Am J Ophthalmol 14:202, 1931 32. Jarrett WH, Gutman FA: Ocular complications of infection in the paranasal sinuses. Arch Ophthalmol 81:683, 1969 33. Hajek M:Complications involving the orbit and visual organ. In Pathology and Treatment of Inflammatory Disease of the Nasal Accessory
Sinuses. vol 2, 1926578–606 34. Silver HS, Fucci MJ, Flanagan JC, Lowry LD: Severe orbital infection as a complication of orbital fracture. Arch Otolaryngol Head Neck Surg 118:845, 1992 35. Westfall CT, Shore JW: Isolated fractures of the orbital floor: risk of infection and the role
of the antibiotic prophylaxis. Ophthalmic Surg 22:409, 1991 36. Goldfarb MS, Hoffman DS, Rosenberg S: Orbital cellulitis and orbital fractures. Ann Ophthalmol 19:97, 1987 37. Kaufman SJ: Orbital mucopyoceles: two cases and a review. Surv Ophthalmol 25:253, 1981 38. Rees TD, Craig SM, Fisher Y: Orbital abscess following blepharoplasty. Plast Reconstr Surg 73:126, 1983 39. Wilson ME, Paul TO: Orbital cellulitis following strabismus surgery. Ophthalmic Surg 18:92, 1987 40. von Noorden GK: Orbital cellulitis following extraocular muscle surgery. Am J Ophthalmol 74:627, 1972 41. Janakarajah N, Sukumaran K: Orbital cellulitis of dental origin: case report and review of the literature. Br J Oral Maxillofac Surg 23:140, 1985 42. Bullock JD, Fleishman JA: The spread of odontogentic infections to the orbit: diagnosis and management. J Oral Maxillofac Surg 43:749, 1985 43. Smith AT, Spencer JT: Orbital complications resulting from lesions of the sinuses. Ann Otol Rhinol Laryngol 57:5, 1948 44. Abbott RL, Shekter WB: Necrotizing erysipelas of the eyelids. Ann Ophthalmol 11:381, 1979 45. Scott PM, Bloome MA: Lid necrosis secondary to streptococcal periorbital cellulitis. Ann Ophthalmol 4:461, 1981 46. Smith TF, O'Day D, Wright PF: Clinical implications of preseptal (periorbital) cellulitis in
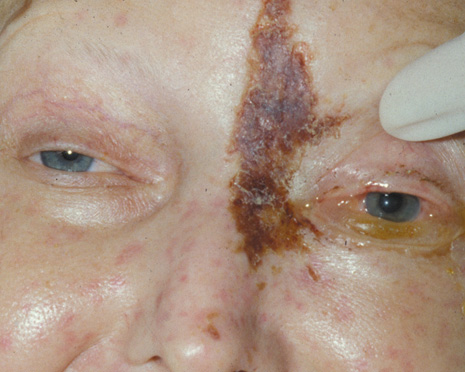
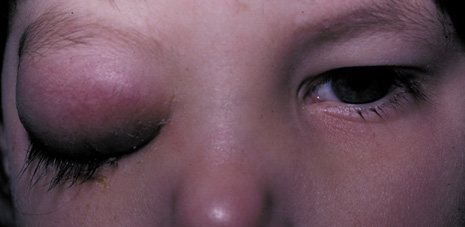
childhood. Pediatrics 62:1006, 1978 47. Bergin DJ, Wright JE: Orbital cellulitis. Br J Ophthalmol 70:174, 1986 48. Slavin ML, Glaser JS: Acute severe irreversible visual loss with sphenoethmoiditis: ‘posterior’ orbital
cellulitis. Arch Ophthalmol 105:345, 1987 49. Grossniklaus HE, Wojno TH: Leukemic infiltrate appearing as periorbital cellulitis. Arch Ophthalmol 108:484, 1990 50. Bach MC, Knowland M, Schuyler WBJ: Acute orbital myositis mimicking orbital cellulitis. Ann Intern Med 109:243, 1988 51. Hornblass A, Herschorn BJ, Stern K, Grimes C: Orbital abscess. Surv Ophthalmol 29:169, 1984 52. Thatcher DB: Necrotic choroidal melanoma presenting with severe inflammation. Surv Ophthalmol 12:247, 1967 53. Shields JA, Shields CL, Suvarnamani C, et al: Retinoblastoma manifesting as orbital cellulitis. Am J Ophthalmol 112:442, 1991 54. Uzcategui N, Warman R, Smith A, Howard CW: Clinical practice guidelines for the management of orbital cellulitis. J Pediatr Ophthalmol Strabismus 35:73, 1998 55. Younis RT, Lazar RH, Bustillo A, Anand VK: Orbital infection as a complication of sinusitis: are diagnostic and treatment
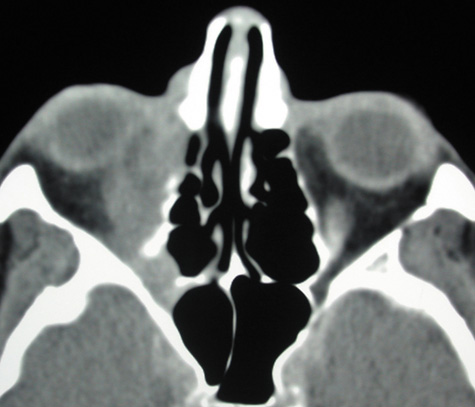
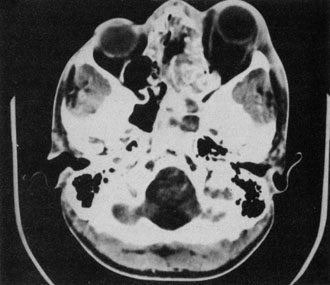
trends changing. Ear, Nose, Throat J 81:771, 2002 56. Harr DL, Quencer RM, Abrams GW: Computed tomography and ultrasound in the evaluation of orbital infection
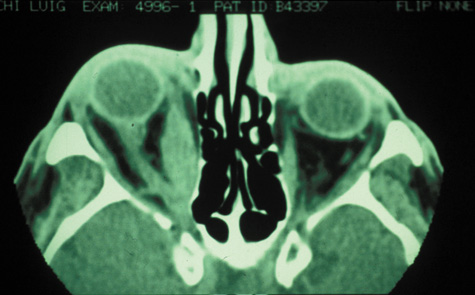
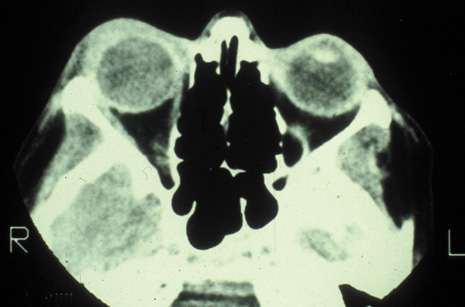
and pseudotumor. Radiology 142:395, 1982 57. Towbin R, Han BK, Kaufman RA, Burke M: Postseptal cellulitis: CT in diagnosis and management. Radiology 158:735, 1986 58. Hirsch M, Lifschitz T: Computerized tomography in the diagnosis and treatment of orbital cellulitis. Pediatr Radiol 18:302, 1988 59. Goldberg F, Berne AS, Oski FA: Differentiation of orbital cellulitis from preseptal cellulitis by computed
tomography. Pediatrics 62:1000, 1978 60. Brenner DJ, Elliston CD, Hall EJ, Berdon WE: Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR 176:289, 2001 61. Carter BL, Bankoff MS, Fisk JD: Computed tomographic detection of sinusitis responsible for intracranial
and extracranial infections. Radiology 147:739, 1983 62. Langham-Brown JJ, Rhys-Williams S: Computed tomography of acute orbital infection: the importance of coronal
sections. Clin Radiol 40:471, 1989 63. Zimmerman RA, Bilaniuk LT: CT of orbital infection and its cerebral complications. AJR 134:45, 198 64. Lemke BN, Gonnering RS, Harris J, Weinstein JM: Orbital cellulitis with periorbital elevation. Ophthalmic Plast Reconstruct Surg 3:1, 1987 65. Karesh J, Lakhanpal V, Haney P, et al: Metastatic anaerobic orbital subperiosteal abscess: value of CT scanning. J Pediatr Ophthalmol Strabismus 19:52, 1982 66. Handler LC, Davey IC, Hill JC, Lauryssen C: The acute orbit: differentiation of orbital cellulitis from periosteal
abscess by computerized tomography. Neuroradiology 33:15, 1991 67. Gold SC, Arrigg PG, Hedges TR: Computerized tomography in the management of acute orbital cellulitis. Ophthalmic Surg 18:753, 1987 68. Patt BS, Manning SC: Blindness resulting from orbital complications of sinusitis. Otolaryngol Head Neck Surg 104:789, 1991 69. Goodwin WJ, Weinshall M, Chandler JR: The role of high resolution computerized tomography and standardized ultrasound
in the evaluation of orbital cellulitis. Laryngoscope 92:728, 1982 70. Saini JS, Mohan K, Khandalavala B: Wooden foreign bodies of the orbit. Orbit 8:139, 1989 71. Casteel I, DeBleecker C, Demaerel P, et al: Orbital myositis following an upper respiratory tract infection: contribution
of high resolution CT and MRI. J Belg Radiol 74:45, 991 72. Galetta SL, Wulc AE, Goldberg HI, et al: Rhinocerebral mucormycosis: management and survival after carotid occlusion. Ann Neurol 28:103, 1990 73. Uehara F, Ohba N: Diagnostic imaging in patients with orbital cellulitis and inflammatory
pseudotumor. Int Ophthalmol Clin 42:133, 2002 74. Frederick J, Braude AI: Anaerobic infection of the paranasal sinuses. N Engl J Med 290:135, 1974 75. Robie F, O'Neal R, Kelsey DS: Periobital cellulitis. J Pediatr Ophthalmol 14:354, 1977 76. Morgan PR, Morrison WV: Complications of frontal and ethmoidal sinusitis. Laryngoscope 90:661, 1980 77. Welsh LW, Welsh JJ: Orbital complications of sinus disease. Laryngoscope 84:848, 1974 78. Quick CA, Payne E: Complicated acute sinusitis. Laryngoscope 82:1248, 1972 79. Noel LP, Clarke WN, Peacocke TA: Periorbital and orbital cellulitis in childhood. Can J Ophthalmol 16:178, 1981 80. Hemady R, Zimmerman A, Katzen BW, Karesh JW: Orbital cellulits caused by Eikenlla corrodens. Am J Ophthalmol 114:584, 1992 81. Eustis HS, Mafee MF, Walton C, Mondonca J: MR imaging and Ct of orbital infections and complications in acute rhinosinusitis. Radiol Clin North Am 36:1165, 1998 82. Siber GR, Schur PH, Aisenberg AC, et al: Correlation between serum IgG-2 concentrations and the antibody response
to bacterial polysaccharide antigens. N Engl J Med 303:178, 1980 83. Robbins JB, Schneerson R, Argaman M, Handzel ZT: Haemophilus influenzae type b: disease and immunity in humans. Ann Intern Med 78:259, 1973 84. Morell A, Skvaril F, Hitzig WH, Barandum S: IgG subclasses: development of the serum concentrations in “normal” infants
and children. J Pediatr 80:960, 1972 85. Schur PH, Rosen F, Norman ME: Immunoglobulin subclasses in normal children. Pediatr Res 13:181, 1979 86. Adams WG, Deaver KA, Cochi SL, et al: Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA 269:221, 1993 87. Antoine GA, Grundfast KM: Periorbital cellulitis. Int J Pediatr Otorhinolaryngol 13:273, 1987 88. Stammberger H: Endoscopic endonasal surgery: concepts in treatment of recurring rhinosinusitis. Otolaryngol Head Neck Surg 94:143, 1986 89. Partamian LG, Jay WM, Fritz KJ: Anaerobic orbital cellulitis. Ann Ophthalmol 15:123, 1983 90. Tannenbaum M, Tenzel J, Byrne SF, et al: Medical management of orbital abscess. Surv Ophthalmol 30:211, 1986 91. Rubin SE, Rubin LG, Zito J, et al: Medical management of orbital subperiosteal abscess in children. J Pediatr Ophthalmol Strabismus Surg 26:21, 1989 92. Catalano RA, Smoot CN: Subperiosteal orbital masses in children with orbital cellulitis: time
for a reevaluation? J Pediatr Ophthalmol Strabismus Surg 27:141, 1990 93. Zhanel GG, Ennis K, Vercaine L, et al: A critical review of the fluoroquinolones. Drugs 62:13, 2002 94. Lasko B, Lau CY, Saint-Pierre C, et al: Efficacy and safety of oral levofloxacin compared with clarithromycin in
the treatment of acute sinusitis in adults: a multicentre, double blind, randomized
study. J Int Med Res 26:281, 1998 95. Barman Balfour JA, Lamb HM: Moxifloxacin, a review of its potential in the management of community-acquired
respiratory tract infections. Drugs 59:115, 2000 96. Chalumeau M, Tonnelier S, d'Athis P, et al: Fluoroquinolone safety in pediatric patients: a prospective, multicenter, comparative
cohort study in France. Pediatrics 111:714, 2003 97. Newell FW, Leveille AS: Management and complications of bacterial periorbital and orbital cellulitis. Metab Pediatr Syst Ophthalmol 6:209, 1982 98. Spires JR, Smith RJH: Bacterial infections of the orbital and perirobital soft tissues in children. Laryngoscope 96:763, 1986 99. Barkin RM, Todd JK: Periorbital cellulitis in children. Pediatrics 62:390, 1978 100. Maniglia AJ, Kronberg FG, Culbertson W: Visual loss associated with orbital and sinus disease. Laryngoscope 94:1050, 1984 101. Manning SC: Endoscopic management of medial subperiosteal orbital abscess. Arch Otolaryngol Head Neck Surg 119:789, 1993 102. Jarrett WH, Gutman FA: Ocular complications of infection in the paranasal sinuses. Arch Ophthalmol 81:683, 1969 103. Forstot SL, Ellis PP: Nontraumatic rupture of the globe secondary to orbital cellulitis. Am J Ophthalmol 88:262, 1979 104. Bhatia K, Jones NS: Septic cavernous thrombosis secondary to sinusitis: are anticoagulants
indicated? A review of the literature. J Laryngol Otol 116:667, 2002 105. Maniglia AJ, Goodwin WJ, Arnold JE, Ganz E: Intracranial abscess secondary to nasal, sinus, and orbital infections
in adults and children. Arch Otolaryngol Head Neck Surg 115:1424, 1989 106. Malani PN, Kauffman CA: Invasive and allergic fungal sinusitis. Curr Infect Dis Rep 4:225, 2002 107. Bailey JC, Fulmer JM: Aspergillosis of the orbit. Am J Ophthalmol 51:670, 1961 108. Zinneman HH: Sinoorbital aspergillosis: report of a case and a review of the literature. Minn Med 55:661, 1972 109. Stringer SP, Ryan MW: Chronic invasive fungal rhinosinusitis. Otolaryngol Clin North Am 33:375, 2000 110. Romett JL, Newman RK: Aspergillosis of the nose and paranasal sinuses. Laryngoscope 92:764, 1982 111. McGill TJ, Simpson G, Healy G: Fulminant aspergillosis of the nose and paranasal sinuses: a new clinical
entity. Laryngoscope 90:748, 1980 112. Huchton DM: Allergic fungal sinusitis. Allergy Asthma Proc 24:307, 2003 113. Hedges TR, Leung LE: Parasellar and orbital apex syndrome caused by aspergillosis. Neurology 26:117, 1976 114. Washburn RG, Kennedy DW, Begley MG, et al: Chronic fungal sinusitis in apparently normal hosts. Medicine 67:231, 1988 115. Zinreich SJ, Kennedy DW, Malat J, et al: Fungal sinusitis: diagnosis with CT and MR imaging. Radiology 169:439, 1988 116. Dortzbach RK, Segrest DR: Orbital aspergillosis. Ophthalmic Surg 14:240, 1983 117. Yu VL, Wagner GE, Shadomy S: Sino-orbital aspergillosis treated with combination anjpgungal therapy: successful
therapy after failure with amphotercin B and surgery. JAMA 244:814, 1980 118. Kitahara M, Seth VK, Medoff G, et al: Activity of amphotericin B, 5-fluorocytosine and rifampin against six clinical
isolates of Aspergillus. Antimicrob Agents Chemother 9:915, 1976 119. Codish SD, Tobias JS, Hannigan MM: Combined amphotericin flucytosine therapy in aspergillosis pneumonia. JAMA 241:2418, 1979 120. Harris GJ, Will BR: Orbital aspergillosis: conservative debridement and local amphotericin
irrigation. Ophthalmic Plast Reconstr Surg 5:207, 1989 121. Schwartz JN, Donnelly EH, Klintworth GK: Ocular and orbital phycomycosis. Surv Ophthalmol 22:3, 1977 122. Pillsbury HC, Fisher ND: Rhinocerebral mucormycosis. Arch Otolaryngol 103:600, 1977 123. Ferry A, Abedi S: Diagnosis and management of rhinoorbitocerebral mucormycosis (phycomycosis): a
report of 16 personally observed cases. Ophthalmology 90:1096, 1983 124. Baum JL: Rhinoorbital mucormycosis occurring in an otherwise apparently healthy
individual. Am J Ophthalmol 63:355, 1967 125. Qingli L, Orcutt JC, Seifter LR: Orbital mucormycosis with retinal and ciliary artery occlusions. Br J Ophthalmol 73:680, 1989 126. Daly AL, Velazquez LA, Bradley SF, Kauffman CA: Mucormycosis: association with deferoxamine therapy. Am J Med 87:468, 1989 127. Bray WE, Gaingiacomo J, Ide CH: Orbital apex syndrome. Surv Ophthalmol 32:136, 1987 128. Bullock JD, Jampol LM, Fezza AJ: Two cases of orbital phycomycosis with recovery. Am J Ophthalmol 78:811, 1974 129. Kohn R, Hepler R: Management of limited rhino-orbital mucormycosis without exenteration. Ophthalmology 92:1440, 1985 130. Couch L, Theilen F, Mader JT: Rhinocerebral mucormycosis with cerebral extension successfully treated
with adjunctive hyperbaric oxygen therapy. Arch Otolaryngol Head Neck Surg 114:791, 1988 |