INFANTILE CAPILLARY HEMANGIOMA
Hamartomas are composed of abnormal mixtures of tissue elements or an abnormal proportion of a single element that is usually present in that site. Benign hemangioendothelioma, strawberry hemangioma or nevus, and infantile or hemangioblastic hemangioma are additional terms used to describe this high-flow hamartomatous proliferation of primitive vasoformative tissues. Infantile capillary hemangioma is the most common orbital vascular tumor of childhood, estimated to occur in 1% to 2% of infants.7,8 It occurs predominantly in female infants, with a 3:2 female-to-male ratio most frequently reported.9,10 Although usually sporadic, rare heritable infantile hemangiomas have been described, and may perhaps be under-reported.11,12 Predisposing factors for the development of capillary hemangiomas include maternal chorionic villus sampling13 and prematurity.14
Clinical Features
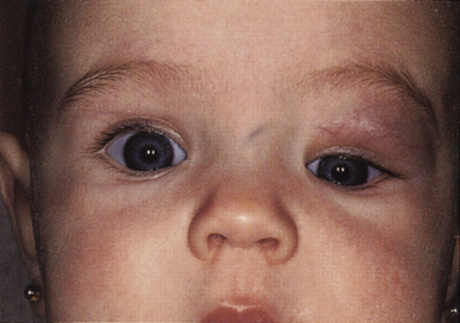
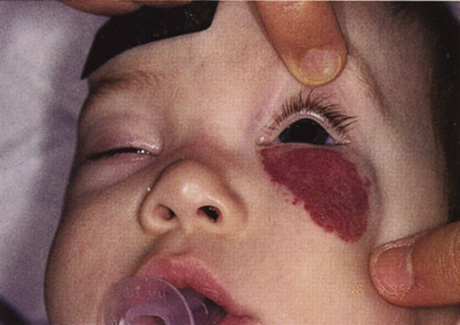
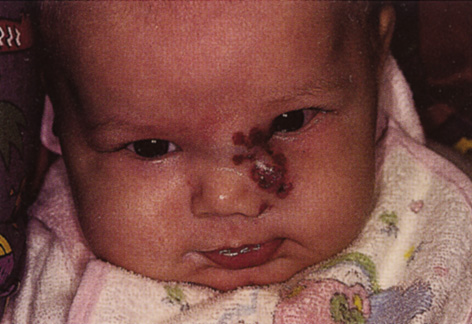
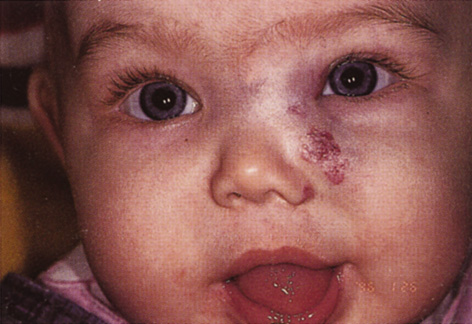
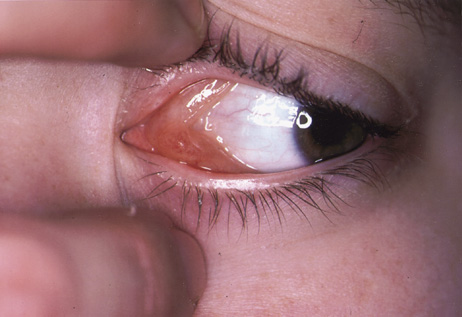
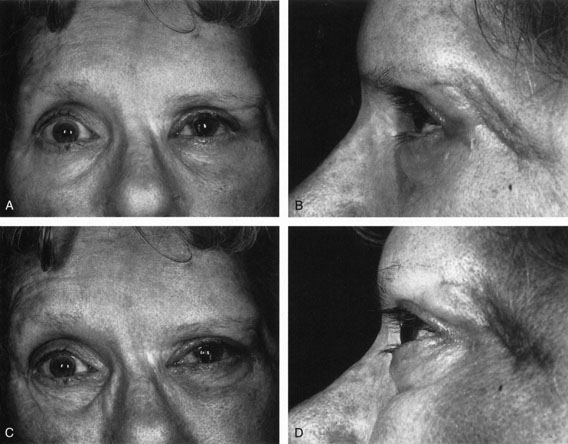
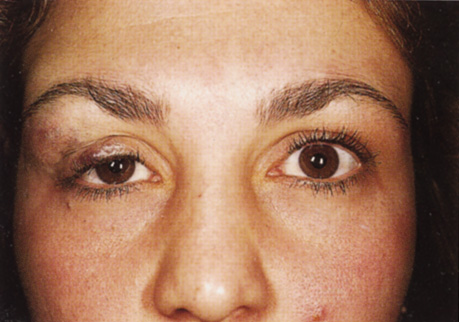
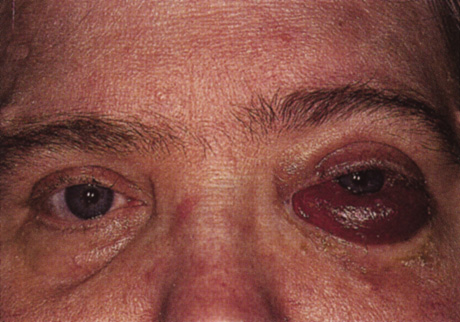
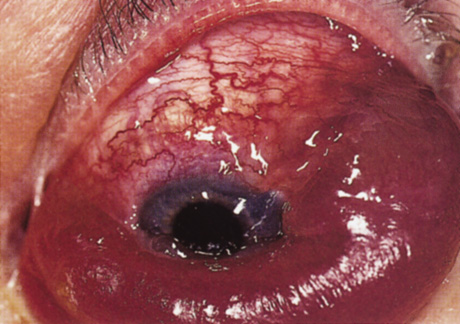
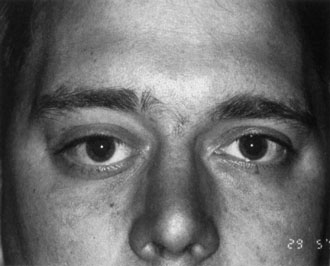
One-third of all orbital capillary hemangiomas are noted at birth, and virtually all are diagnosed by age 6 months.10 Most commonly a unilateral diffuse subcutaneous or circumscribed, dimpled, red dermal lesion (strawberry nevus) is noted on the upper eyelid (Figs. 1 and 2).9,10 In more than one-third of patients, the palpebral or fornical conjunctiva is involved.10 Commonly, this superficial lesion is associated with a component deep to the orbital septum resulting in variable degrees of proptosis in 38% of patients.10 More rarely, it can occur as an isolated lesion posterior to the orbital septum without a superficial component: In Haik and colleagues' large series of 101 infants with capillary hemangiomas, seven presented with proptosis alone.10 Forty-six percent of patients have an increase in lesion size with crying or Valsalva maneuver.10 Very rarely, hemangiomas can occur within the extraocular muscles.15 Early flat, circumscribed lesions rapidly expand into bulky, compressible masses over a period of weeks to months as the capillary lumina of the endotheliomatous hamartoma opens.16 This distinguishes the capillary hemangioma from the port-wine stain (nevus flammeus) associated with the Sturge-Weber and Klippel-Trenaunay syndromes, which remains flat to slightly thickened and is noncompressible. The infantile capillary hemangioma usually reaches its largest size within 6 months and then typically involutes.10,17 Fine, stellate areas of pale scarring (herald spots) appear during resolution (Fig. 3). Residual pigmentary skin changes and superficial cutaneous scarring may result.10 The most common ocular complication of adnexal capillary hemangiomas is visual loss, most often resulting from amblyopia or in rare cases optic atrophy. Amblyopia occurs in 44% to 64% of these infants and usually is a result of anisometropia, visual deprivation, or both.9,10,18 Anisometropia may result from axial myopia induced by the eyelid closure or astigmatism (plus cylinder axis points toward the tumor mass).19 Strabismus may be present in up to 34% of patients with periorbital infantile capillary hemangioma.18 Associated dermal or visceral hemangiomas throughout the body have been reported in 29% of infants with periorbital capillary hemangiomas.10 Laryngeal hemangiomas are the most frequent visceral vascular manifestation. In rare cases, hepatic, gastrointestinal, or intracerebral hemangiomas may be present.20 Sequestration of platelets and red blood cells leading to thrombocytopenia and bleeding diathesis (Kasabach-Merritt syndrome) can occur with large visceral lesions but are rare with isolated head and neck lesions.10,21
Investigations
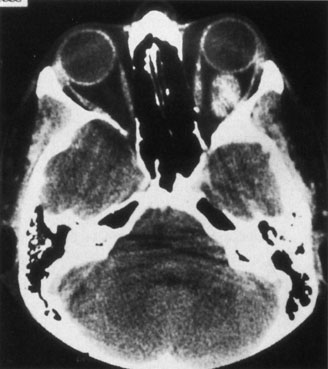
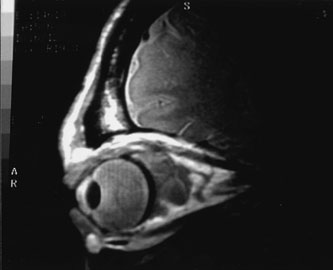
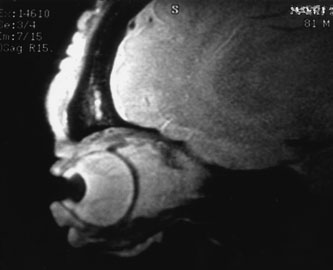
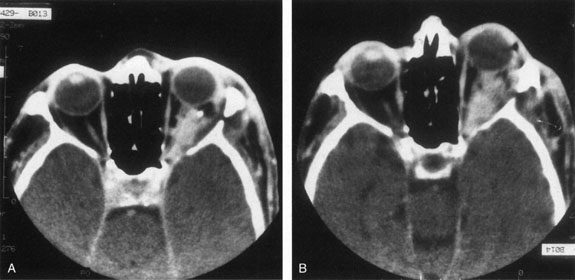
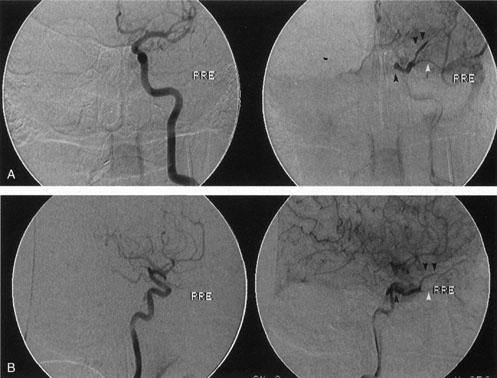
Most capillary hemangiomas can be diagnosed readily by clinical inspection. However, if delineation of the extent of deep orbital involvement is required, or the diagnosis is unclear, magnetic resonance imaging (MRI) with surface coils, gadolinium–pentetic acid enhancement, and fat suppression (to detect enhancement against the orbital fat) is indicated. The tumor is isointense to muscle and gray matter on T1- and hyperintense on T2-weighted images.22 The enhancement seen with intravenous contrast varies from moderate to intense and may be homogeneous or inhomogeneous.10 Lesions undergoing involution are less intense and more inhomogeneous. Major feeding vessels appear as black, serpiginous structures because of the “flow void” phenomenon.22 B-scan ultrasonography shows a smooth, lobular, or irregular mass with variable internal reflectivity that blends into surrounding orbital structures.23 A-scan ultrasonography shows alternating high internal reflectivity (high echo spikes) and low internal reflectivity (low echo spikes) resulting from the variable architecture and acoustic interfaces of vascular spaces, cellular areas, and septa, and moderate sound attenuation.24 High vascular flow may be demonstrated on Doppler echography. On computed tomography (CT) scan, the margins of deep infantile hemangiomas vary from moderately well defined to irregular.25 They can occur anywhere within the orbit and may be both intraconal and extraconal. There are no calcifications within these lesions. Occasionally, they indent the globe and are associated with bony orbital enlargement without erosion.10 Very rarely, angiography may be needed in the unusual situation where hazardous superselective embolization is used for life-threatening hemangiomas unresponsive to other therapies.20 These lesions frequently have multiple feeding vessels from both the internal and external carotid arteries.
Histopathology
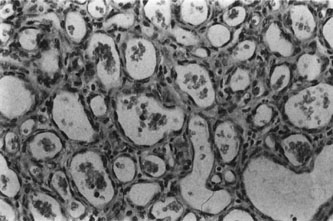
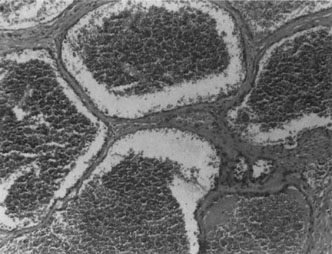
Infantile capillary hemangiomas have a characteristic histopathologic pattern with masses of plump proliferating endothelial cells organized into a network of basement membrane–lined vascular channels with small, irregular lumina (Fig. 4). No true capsule exists.10 A reticulin stain may help delineate primitive vascular structures in early stages of proliferation when there are few vascular spaces.10 During the proliferative phase or for poorly differentiated tumors, the localization of von Willebrand factor produced by the endothelial cells by either peroxidase or fluorescent antibody technique may prove useful.10 Lesions undergoing involution are characterized by fewer endothelial cells, larger and less numerous vascular channels, increasing collagen deposition, intralesional fat, and in some instances, inflammatory cell infiltrates. The late regression phase is dominated by fibrosis.10
Differential Diagnosis
The differential diagnosis of a rapidly expanding orbital mass in infancy and early childhood includes rhabdomyosarcoma, chloroma, neuroblastoma, vascular malformations, congenital hydrops of the nasolacrimal sac, dermoid cyst, and orbital cellulitis.
Management Natural History
Outlining the natural history of these lesions to the parents is extremely important in alleviating their concerns and anxieties and in justifying the usual decision for conservative management. The rate of complete resolution without treatment is 32% to 60% at 4 years of age, and 72% to 76% at 7 years of age; there is variable improvement in the remaining children until the age of 10 to 12 years.17
Treatment
Treatment is required for children with refractory amblyopia, threatened occlusion of the visual axis, compression of the optic nerve, or corneal exposure secondary to severe proptosis. Controversy exists about the most appropriate management of induced astigmatism and anisometropic refractive error, with some authors advocating early treatment to reverse severe refractive errors.10,26 Nonvisual indications for therapy include deep lesions that bleed frequently, secondary maceration and erosion of the epidermis, or severe disfigurement. Systemic indications for treatment are cardiovascular, hematologic, or obstructive complications.20 Intralesional corticosteroids have been the mainstay of initial therapy in lesions requiring treatment. Their therapeutic effectiveness results from arteriolar constriction and narrowing of precapillary sphincters.27 Combinations of short- and long-acting corticosteroids of various doses have been successful. Generally, the long-acting depot steroid is directed deep into the lesion to prevent deposits from being visible under the skin, and the more soluble short-acting corticosteroid is given subcutaneously around the periphery of the capillary hemangioma. Injections usually are given under mask inhalation; separate 1- to 3-mL syringes with 25- or 27-gauge needles are used for each substance. The following are two popular regimens: (a) 40 mg triamcinolone acetate and 6 mg betamethasone given separately into the lesion28 or (b) 40 mg methylprednisolone and 4 mg dexamethasone sodium phosphate given separately into the lesion.9 Some authors vary the dose according to the size of the capillary hemangioma.29,30 Involution of the tumor may begin several days after injection and usually is considerable within 2 to 4 weeks.26 Injections can be repeated at 6-week intervals, as needed.9,30 Intralesional corticosteroids are effective in inducing moderate to marked involution in 45% to 88% of cases,20,26,30 but recurrence and regrowth with diminution of the steroid dose is not infrequent. Potential complications of intralesional steroids include adrenal suppression, blindness secondary to retinal embolization of the corticosteroid, eyelid depigmentation, eyelid necrosis, and subcutaneous fat atrophy.31–34 Other treatment modalities include topical steroids, sub-Tenon infusion of steroids, systemic corticosteroids, laser therapy, interferon injections, selective arterial embolization, and surgery.20,35–37 Topical clobetasol propionate cream given twice daily for 2 weeks, with 1 week drug-free periods, may be an alternative mode of treatment in those patients whose parents decline intralesional steroids.37 Promising results without fibrosis or hypertrophic scarring have been reported with repeated treatments using the flashlamp pumped-pulsed dye lasers, particularly if initiated early, when lesions are relatively flat.38 Interferon alfa, which inhibits both endothelial cell and fibroblast proliferation, has been used for life-threatening hemangiomas resistant to corticosteroids.39,40 Hastings and colleagues examined 40 patients aged 2 to 36 months with life- or organ-threatening hemangiomas treated with daily subcutaneous interferon alfa-2b for 3 months, followed by taper or retreatment. An average 82% reduction in tumor volume occurred, with clinical response observed at an average of 6 weeks.40 Therapeutic arterial embolization is particularly hazardous and has yielded inconsistent results; it is therefore a last resort for life-threatening situations.20 Early surgical intervention in infants 2 to 20 months for selected hemangiomas without a significant cutaneous component has been reported with good results.25,35,41 Preoperative radiologic tests (magnetic resonance angiogram, arteriogram, or digital intravenous arteriogram) and blood typing are advisable for all patients. A true capsule is not present,35 making complete surgical excision of extensive infiltrating lesions very difficult. When surgery is performed in patients with Kasabach-Merritt syndrome (very large platelet-consuming lesions), systemic antifibrinolytic agents are needed, including aminocaproic acid or tranexamic acid. Cryotherapy, irradiation, and intralesional sclerosing agents have largely been abandoned because of inadequate effectiveness or unacceptable side effects
CAVERNOUS HEMANGIOMA
Cavernous hemangiomas are benign, noninfiltrative, low-flow hamartomas. Although often cited as the most common primary orbital tumor of adults,8,10 in the largest reported series from Moorfields Eye Hospital, cavernous hemangiomas constituted only 7% of 1,270 patients with primary orbital tumors and were outnumbered by venous flow malformations (primary orbital varices) by a ratio of 3:1.42
Clinical Features
Patients typically present in the fourth and fifth decades, although the age range spans from occasional case reports in children younger than 18 years of age43 to an adult 78 years old.44 A 60% to 70% female preponderance is reported consistently.10,42,45 Seventy percent of patients present with gradually increasing painless proptosis, which usually is axial.10,45 Less common symptoms of orbital pain, eyelid swelling, diplopia, and gaze-induced amaurosis can occur.42,45–47 In a series of 162 cavernous hemangiomas (110 histologically proven), no episodes of acute or subacute hemorrhage were documented.48 Cavernous hemangiomas appear to enlarge by proliferation of capillaries,10 and local hemodynamic disturbances, hypoxia, and hormonal changes during pregnancy may stimulate their growth.10,44,49On clinical examination, other signs of mass effect may accompany the proptosis: In their series of 85 patients, McNab and Wright reported 32% with posterior pole choroidal striae and 16% with compressive optic neuropathy.42 Orbital cavernous hemangiomas usually are isolated lesions.45 Very rarely, they may be associated with multiple hemangiomas involving predominantly the arms and trunk (usually evident at birth) and visceral lesions commonly in the small intestine, leading to gastrointestinal bleeding and iron deficiency anemia (the blue rubber bleb nevus syndrome).50,51 This is usually a sporadic syndrome, although pedigrees with autosomal-dominant inheritance have been reported.52
Investigations
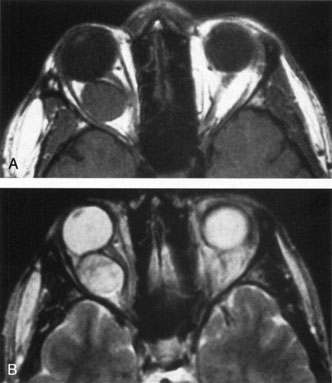
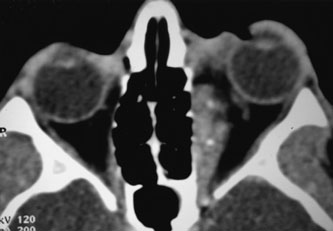
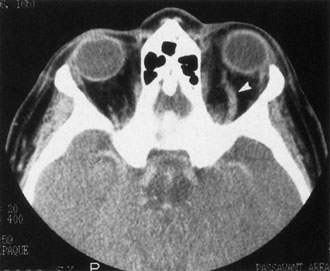
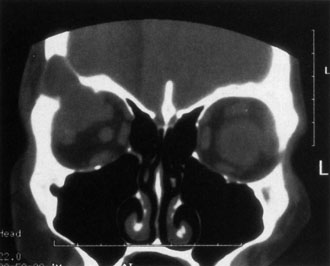
Contrast-enhanced CT of the orbits with direct and coronal cuts is the radiologic study of choice for these patients.45 A well-demarcated, encapsulated oval or rounded mass is revealed, which is typically intraconal in the lateral part of the middle third of the orbit (Fig. 5) but occasionally may extend to the extraconal space.42 Septa within the tumor may be apparent on high-resolution CT. The posterior pole of the globe frequently is indented by the rounded anterior margin of the tumor.42 The optic nerve typically is displaced rather than surrounded by the tumor. Subtle outward bowing of the lateral orbital wall or increase in orbital size may be present, consistent with a long-standing, slowly growing mass lesion.10,42 Enhancement with intravenous contrast occurs and may be homogeneous or inhomogeneous.10,45 Rarely, cavernous hemangiomas may occur as an intraosseous tumor within the orbital or facial bones.42,53–55 Although usually an isolated intraorbital lesion, multiple lesions in one orbit occurred in 8 of 164 (5%) patients of three combined large studies,10,42,45 and bilateral multiple cavernous hemangiomas also have been described.56 In contrast to patients with venous flow malformations in which phleboliths are common, phleboliths are rare in cavernous hemangiomas. Three large studies comprising 164 patients with cavernous hemangioma all reported that no calcification was detected within the tumor.10,42,45If the diagnosis is still unclear or if better definition of details and localization of the lesion is required, then MRI should be performed. Magnetic resonance imaging demonstrates nonspecific characteristics of a lesion isointense to muscle and gray matter on T1-weighted images and hyperintense on T2-weighted images (Fig. 6).57 The lesions show initial central patchy enhancement, which fills up homogeneously within 20 to 60 minutes.58 If ultrasonography is performed, B-scan ultrasonography shows a well-circumscribed mass with a sharply defined anterior acoustic border.23 A-scan ultrasonography shows high reflectivity of the echo signals resulting from the multiple blood-filled vascular channels, regular internal structure with a higher anterior and posterior spike marking the capsule, and moderate sound attenuation (angle of decrease of the echo spike within the lesion).24 Arteriography is not indicated.
Histopathology
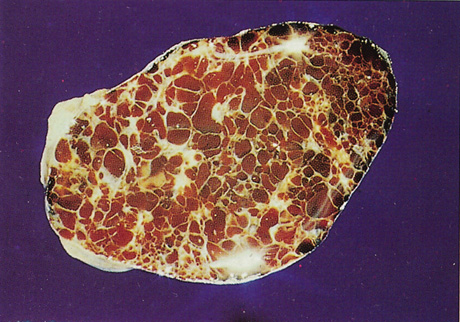
Histopathology reveals large, endothelium-lined, blood-filled spaces separated by fibrous septa and surrounded by a fine capsule (Figs. 7 and 8). Abundant, loosely distributed smooth muscle is present in the vascular walls, and scattered inflammatory cells may be seen.10,44 Histopathologic confirmation of the low-flow character of this lesion is seen by the presence of menisci in some vascular spaces and evidence of decomposed blood.
Differential Diagnosis
The differential diagnosis of cavernous hemangioma includes neurofibroma, cystic schwannoma, fibrous histiocytoma, and vascular leiomyoma, which all can have identical clinical, CT, and MRI findings. Hemangiopericytoma, hemangioendothelioma, and cystic schwannoma can have similar ultrastructural features.45 Osseous hemangioma usually can be distinguished from meningioma by the lack of extensive hyperostotic changes and from osteosarcoma, multiple myeloma, and metastatic cancer by the absence of irregular bone destruction.53
Management Natural History
As the natural history of cavernous hemangiomas is to enlarge, some authors advise excision as soon as the diagnosis is established,1,59 whereas others believe that observation of asymptomatic cavernous hemangiomas is reasonable.10
Treatment
Treatment, when indicated, involves surgical excision of the lesion. Blunt dissection reveals a plump, nodular, plum-colored, encapsulated mass with vascular channels on its well-defined surface. Because of its low-flow character, a cavernous hemangioma can be punctured during surgery, leading to exsanguination and shrinkage of the tumor, which facilitates its removal.42 A cryoprobe may assist with extraction. If an apical vascular tag can be identified, it should be cauterized; otherwise the gush of blood when it is transected can be controlled with gentle tamponade. There is no evidence of recurrence from incompletely excised lesions.10 The risks of surgery depend on the location of the hemangioma within the orbit: Removal at the orbital apex can be challenging. Although the transcranial approach is optimal for tumors of the superomedial orbital apex, other apical locations can be approached transorbitally.60 Goldberg and colleagues reported no visual loss and no dysmotility in two patients with inferior and superolateral apical hemangiomas resected transorbitally.60 Two of four apical tumors operated on by Missori and colleagues via a transcranial approach developed “complete amaurosis.”61 Reports of postoperative complications from nonapical tumors vary greatly. Lumping together four apical and 21 nonapical intraconal hemangiomas, Missori and colleagues found no complications in the two patients operated on transorbitally, but permanent ptosis in 3/23 (13%), permanent “visual worsening” in 8/23 (62%), and ophthalmoplegia in 4/23 (17%) of patients operated on transcranially.61 Kersten and Kulwin described 37 surgeries for removal of orbital hemangiomas (location within orbit not given); 35 removed via orbitotomy and two via craniotomy. One patient suffered “reduction in visual acuity,” and limitation of extraocular motility occurred in two patients.62 Gdal-On and Gelfand reported on 12 patients with hemangiomas confined mainly to the middle third of the orbit (no apical tumors) undergoing cryosurgical extraction from a 360-degree conjunctiva peritomy. One patient lost vision because of intraoperative cilioretinal arterial occlusion; there were no complications in the remaining 11 patients.63 Pelton and Patel described five intraconal cavernous hemangiomas removed via a superomedial lid crease approach. One patient had transient vertical diplopia for 2 months, and two patients had transient ptosis for 2 and 6 weeks.64