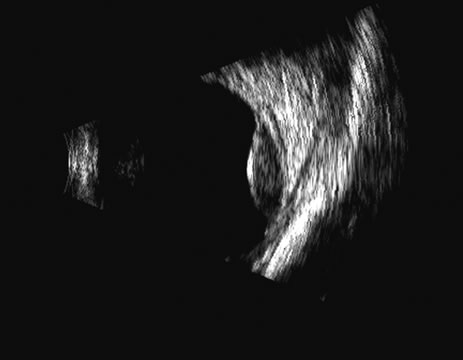
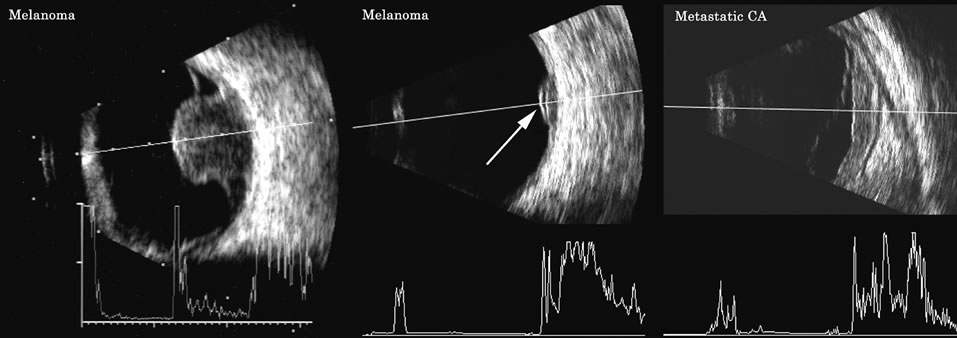
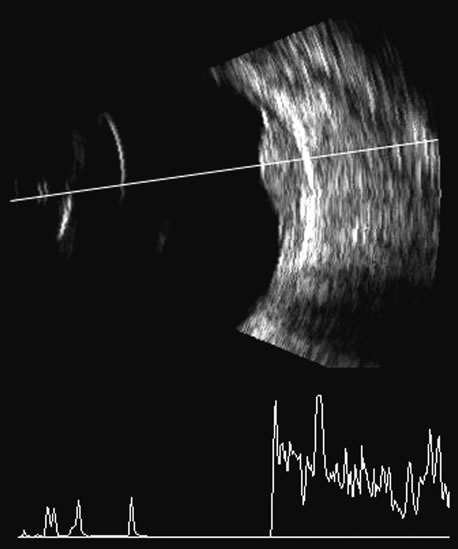
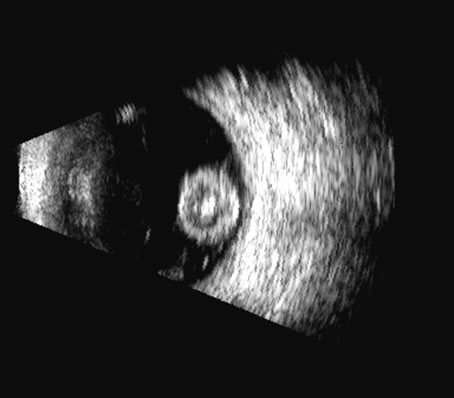
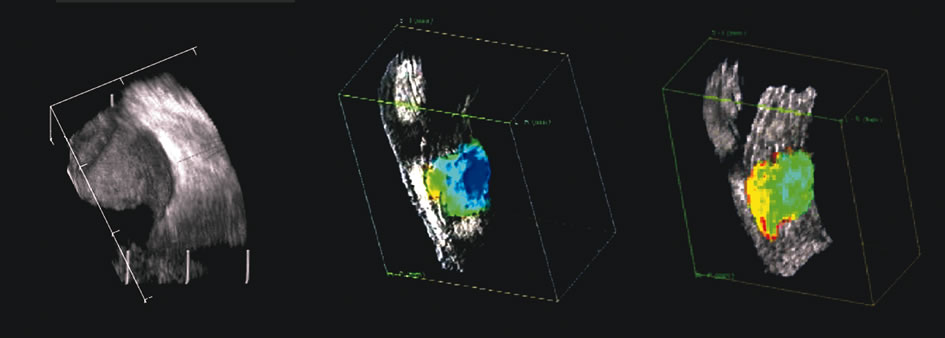
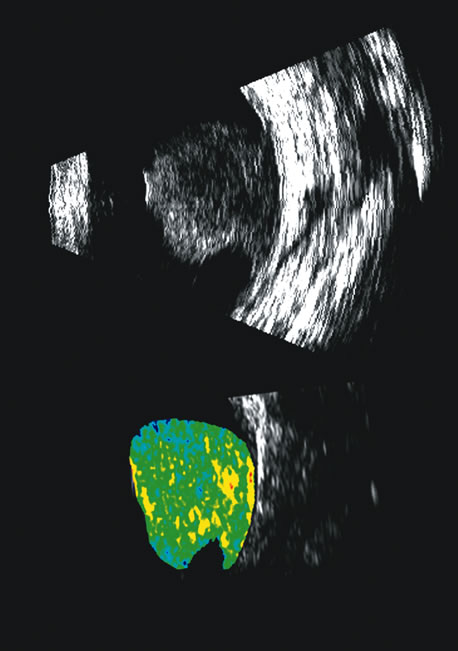
1. Coleman DJ, Silverman RH, Daly SM, Rondeau MJ: Advances in ophthalmic ultrasound. Radiol Clin North Am 36:1073, 1998 2. Coleman DJ, Lizzi FL, Jack RL: Ultrasonography of the eye and orbit. Philadelphia, Lea & Febiger, 1977 3. Coleman DJ, Dallow RL, Smith ME: Immersion ultrasonography: Simultaneous A-scan and B-scan. Int Ophthalmol Clin 19:67, 1979 4. Pavlin CJ, Harasiewicz K, Sherar MD, et al: Clinical use of ultrasound biomicroscopy. Ophthalmology 98:287, 1991 5. Pavlin CJ: Practical application of ultrasound biomicroscopy. Can J Ophthalmol 30:225, 1995 6. Pavlin CJ, Foster FS: Ultrasound biomicroscopy. High-frequency ultrasound imaging of the
eye at microscopic resolution. Radiol Clin North Am 36:1047, 1998 7. Foster FS, Pavlin CJ, Harasiewicz KA, et al: Advances in ultrasound biomicroscopy. Ultrasound Med Biol 26:1, 2000 8. Coleman DJ, Franzen LA: Vitreous surgery: Preoperative evaluation and prognostic value of ultrasonic
display of vitreous hemorrhage. Arch Ophthalmol 92:375, 1974 9. Kim DY, Reinstein DZ, Silverman RH, et al: Very high frequency ultrasound analysis of a new phakic posterior chamber
intraocular lens in situ. Am J Ophthalmol 125:725, 1998 10. Liu W, Wu Q, Huang S, et al: [Application of ultrasound biomicroscopy in diagnosis of anterior
segment vitreoretinal disorders]. Yen Ko Hsueh Pao [Eye Science] 13:192, 1997 11. Coleman DJ: Evaluation of ciliary body detachment in hypotony. Retina 15:312, 1995 12. Coleman DJ, Wilcox LM: The choroid: Its function, evaluation, and surgical management. In: Symposium on Medical and Surgical Diseases of the Retina and Vitreous. Transactions
of the New Orleans Academy of Ophthalmology, pp 1–24. St. Louis, CV Mosby, 1983 13. Coleman DJ, Smith ME: Ultrasonic criteria for surgically salvageable pre-phthisical eyes. In White D, Lyons EA (eds): Ultrasound in Medicine, pp 297–298. Vol 4. Proceedings of the 22nd Annual Meeting of the American Institute of Ultrasound
in Medicine. New York, Plenum Press. 1978 14. Coleman DJ, Jack RL: B-scan ultrasonography in diagnosis and management of retinal detachments. Arch Ophthalmol 90:29, 1973 15. Bronson NR, Fisher YL, Pickering NC: Ophthalmic Contact B-Scan Ultrasonography for the Clinician. Westport, CT, International Publication, 1976 16. Downey DB, Nicolle DA, Levin MF, Fenster A: Three-dimensional ultrasound imaging of the eye. Eye 10:75, 1996 17. Coleman DJ, Daly SW, Atencio A, et al: Ultrasonic evaluation of the vitreous and retina. Semin Ophthalmol 13:210, 1998. 18. Wu G, Silverman RH, Coleman DJ, et al: In vivo thickness of human detached retina by ultrasonic signal processing. Graefes Arch Clin Exp Ophthalmol 227:21, 1989 19. Coleman DJ, Rondeau MJ: Diagnostic imaging of ocular and orbital trauma. In Shingleton BJ, Hersh PS, Kenyon KR (eds): Eye Trauma, pp 25–40. St. Louis, Mosby-Year Book, 1991 20. Clemens S, Kroll P, Rochels R: Ultrasonic findings after treatment of retinal detachment by intravitreal
silicone instillation. Am J Ophthalmol 98:369, 1984 21. Jannson F, Sundmark E: Determination of the velocity of ultrasound in ocular tissues at different
temperatures. Acta Ophthalmol (Copenh) 39:899, 1961 22. Jannson F, Koch E: Determination of the velocity of ultrasound in human lens and vitreous. Acta Ophthalmol (Copenh) 40:420, 1962 23. Coleman DJ, Lizzi FL, Franzen LA, Abramson DH: Determination of the velocity of ultrasound in cataractous lenses: Ultrasonography
in ophthalmology. Bibl Ophthalmol 83:246, 1975 24. Thijssen JM, Mol HJM, Timmer MR: Acoustic parameters of ocular tissues. Ultrasound Med Biol 11:157, 1985 25. Coleman DJ: Echographic and histologic tumor height measurements in uveal melanoma [Letter]. Am J Ophthalmol 101:124, 1986 26. Nicholson DH, Frazier-Byrne S, Chiu MT: [Reply to letter]. Am J Ophthalmol 101:125, 1986 27. Coleman DJ, Rondeau MJ, Silverman RH, et al: Computerized ultrasonic biometry and imaging of intraocular tumors for
the monitoring of therapy. Trans Am Ophthalmol Soc 85:49, 1987 28. Ossoinig KC: Standardized echography: Basic principles, clinical applications, and results. Int Ophthalmol Clin 19:127, 1979 29. Ursea R, Coleman DJ, Silverman RH, et al: Correlation of high-frequency ultrasound backscatter with tumor
microstructure in iris melanoma. Ophthalmol 105:906, 1998 30. Feleppa EJ, Lizzi FL, Coleman DJ, et al: Diagnostic spectrum analysis in ophthalmology: A physical perspective. Ultrasound Med Biol 12:623, 1986 31. Coleman DJ, Lizzi FL: Computer-processed acoustic spectral analysis of ophthalmic tissues. Trans Am Acad Ophthalmol Otolaryngol 83: 725, 1977 32. Coleman DJ, Lizzi FL: Computerized ultrasonic tissue characterization of ocular tissues. Am J Ophthalmol 96:165, 1983 33. Green RL, Byrne SF: Diagnostic ophthalmic ultrasound. In Ryan SJ (ed): Retina, pp 191–273. Vol 1. St. Louis, CV Mosby, 1989 34. Shammas HJ: Atlas of Ophthalmic Ultrasonography and Biometry, p 68. St. Louis, CV Mosby, 1984 35. Nicholson DH, Frazier-Byrne S, Chiu MT: Echographic and histologic tumor height measurements in uveal melanoma. Am J Ophthalmol 100:454, 1985 36. Coleman DJ, Abramson DH, Jack RL, et al: Ultrasonic diagnosis of tumors of the choroid. Arch Ophthalmol 91:344, 1974 37. Cusumano A, Coleman DJ, Silverman RH, et al: Three-dimensional ultrasound imaging. Clinical applications. Ophthalmology 105:300, 1998 38. Lee W, Kirk J, Comstock C, Romero R: Vasa previa: Prenatal detection by three-dimensional ultrasonography. Ultrasound Obstet Gynecol 16:384, 2000 39. Lee W, Kirk J, Shaheen K, et al: Fetal cleft lip and palate detection by three-dimensional ultrasonography. Ultrasound Obstet Gynecol 16:314, 2000 40. Lee W, McNie B, Chaiworapongsa T, et al: Three-dimensional ultrasonographic presentation of micrognathia. J Ultrasound Med 21:775, 2002 41. Fisher Y, Hanutsaha P, Tong S, et al: Three-dimensional ophthalmic contact B-scan ultrasonography
of the posterior segment. Retina 18:251, 1998 42. Atta HR: New applications in ultrasound technology. Br J Ophthalmol 83:1246, 1999 43. Basset O, Gimenez G, Mestas JL, et al: Volume measurement by ultrasonic traverse or sagittal cross-sectional
scanning. Ultrasound Med Biol 17:291, 1991 44. Kidd MN, Lyness RW, Patterson CC, et al: Prognostic factors in malignant melanoma of the choroid: A retrospective
survey of cases in Northern Ireland between 1965 and 1980. Trans Ophthalmol Soc UK 105:114, 1986 45. Silverman RH, Coleman DJ, Lizzi FL, et al: In-vivo volume determination by ultrasound. Invest Ophthalmol (Suppl) 32:1194, 1991 46. Coleman DJ, Lizzi FL, Silverman RH, et al: A model for acoustic characterization of intraocular tumors. Invest Ophthalmol Vis Sci 26:545, 1985 47. Coleman DJ, Silverman RH, Rondeau MJ, et al: Correlations of acoustic tissue typing of malignant melanoma and histopathologic
features as a predictor of death. Am J Ophthalmol 110:380, 1990 48. Silverman RH, Folberg R, Boldt HC, et al: Correlation of ultrasound parameter imaging with microcirculatory patterns
in uveal melanomas. Ultrasound Med Biol 23:573, 1997 |