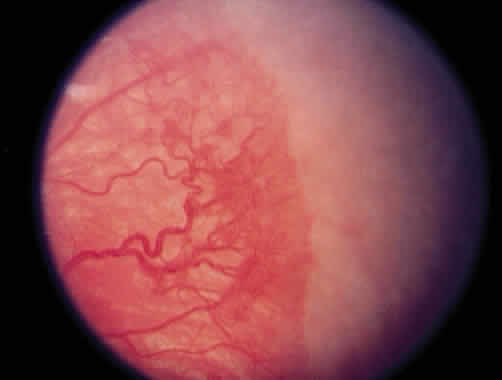
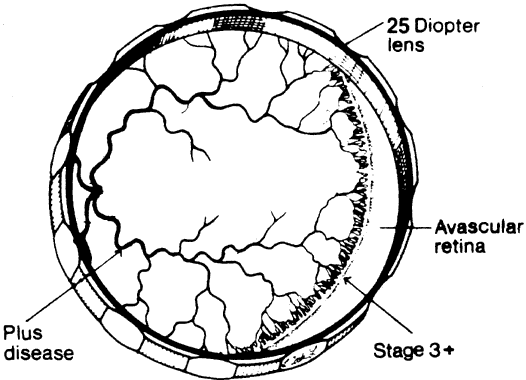
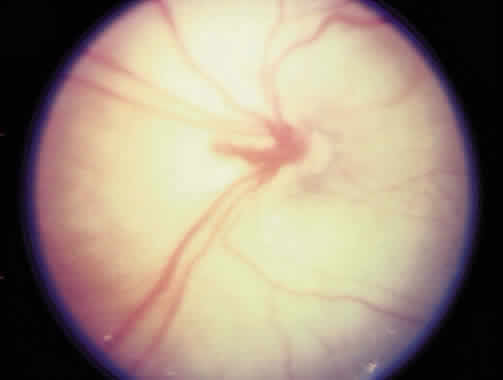
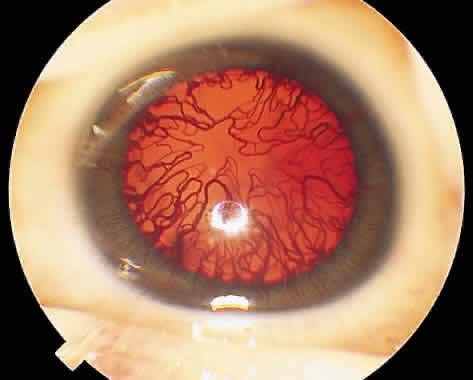
1. Terry T: Extreme prematurity and fibroplastic overgrowth of persistent vascular
sheath behind each crystalline lens: I. Preliminary report. Am J Ophthalmol 25:203, 1942 2. James L, Lanman J: History of oxygen therapy and retrolental fibroplasia. Pediatrics 57:590, 1976 3. Owens W, Owens E: Retrolental fibroplasia in premature infants: II. Studies on prophylaxis
of disease, use of alpha-tocopheral acetate. Am J Ophthalmol 32:1631, 1949 4. Kline B: Histopathologic aspects of retrolental fibroplasia. Arch Ophthalmol 41:553, 1949 5. Kinsey V, Zacharias L: Retrolental fibroplasia incidence in different localities in recent years
and a correlation of the incidence with treatment given the infants. JAMA 139: 572, 1949 6. Campbell K: Intensive oxygen therapy as a possible cause of retrolental fibroplasia: A
clinical approach. Med J Aust 2:48, 1951 7. Crosse V, Evans P: Prevention of retrolental fibroplsia. Arch Ophthalmol 48:83, 1952 8. Ashton N, Ward B, Serpell G: Role of oxygen in genesis of retrolental fibroplasia: Preliminary report. Br J Ophthalmol 49:225, 1953 9. Patz A: Mead Johnson award address: The role of oxygen in retrolental fibroplasia. Pediatrics 19:504, 1957 10. Patz A, Hoech L, de la Cruz E: Studies on the effect of high oxygen administration in retrolental fibroplasia: I. Nursery
observations. Am J Ophthalmol 35:1248, 1952 11. Lanman J, Guy L, Dancis J: Retrolental fibroplasia and oxygen therapy. JAMA 155:223, 1954 12. Kinsey V: Retrolental fibroplasia: Cooperative study of retrolental fibroplasia and
the use of oxygen. Trans Assoc Acad Ophthalmol Otolaryngol 59:15, 1955 13. Cross K: Cost of preventing retrolental fibroplasia? Lancet 2:954, 1973 14. American Academy of Pediatrics Committee on Fetus and Newborn: History
of oxygen therapy and retrolental fibroplasia. Pediatrics 57(Suppl):591, 1976 15. Phelps D: Retinopathy of prematurity: An estimate of vision loss—1979. Pediatrics 67:924, 1981 16. Phelps D: Vision loss due to retinopathy of prematurity. Lancet i:606, 1981 17. Hack M, Fanaroff A: Outcomes of extremely low birth weight infants between 1982 and 1988. N Engl J Med 321:1642, 1989 18. Campbell P, Bull M, Ellis F: Incidence of retinopathy of prematurity in a tertiary newborn intensive
care unit. Arch Ophthalmol 101:1686, 1983 19. Flynn J, Bancalari E, Bawol R et al: Retinopathy of prematurity: A randomized, prospective trial of transcutaneous
oxygen monitoring. Ophthalmology 94:630, 1987 20. The Committee for the Classification of Retinopathy of Prematurity: An
international classification of retinopathy of prematurity. Arch Ophthalmol 102:1130, 1984 21. Committee on Fetus and Newborn: Vitamin E and the prevention of retinopathy
of prematurity. Pediatrics 76: 315, 1985 22. Reynolds J, Hardy R, Kennedy K et al: Lack of efficacy of light reduction in preventing retinopathy of prematurity. N Engl J Med 338:1572, 1998 23. Smith L: VEGF and ROP: Pathophysiology and treatment. International Congress
of Opthalmology, Amsterdam, Holland, June 22, 1998 24. Gilbert C, Canovas R, Kocksch R et al: Causes of blindness and severe visual impairment in children in Chile. Dev Med Child Neurol 36:334, 1994 25. Gilbert C, Foster A: Causes of blindness in children attending four schools for the blind in
Thailand and the Phillippines: A comparison between urban and rural blind
school populations. Int Ophthalmol 17:229, 1993 26. O'Sullivan J, Gilbert C, Foster A: The causes of childhood blindness in South Africa. S Afr Med J 87:1691, 1997 27. Gilbert C, Rahi J, Eckstein M et al: Retinopathy of Prematurity in Middle Income Countries. Lancet 350:12, 1997 28. Palmer E, Flynn J, Hardy R et al: Incidence and early course of retinopathy of prematurity. Ophthalmology 98: 1628, 1991 29. Tasman W: The natural history of active retinopathy of prematurity. Ophthalmology 91:1499, 1984 30. Flynn J, Bancalari E, Bachynski B et al: Retinopathy of prematurity: Diagnosis, severity, and natural history. Ophthalmology 94:620, 1987 31. Schaffer D, Quinn G, Johnson L: Sequelae of arrested mild retinopathy of prematurity. Arch Ophthalmol 102:373, 1984 32. Kingham J: Acute retrolental fibroplasia. Arch Ophthalmol 95:39, 1977 33. Reese A, King M, Owens W: A classification of retrolental fibroplasia. Am J Ophthalmol 36:1333, 1953 34. Cantolino S, Curran J, Van Caden T et al: Acute retrolental fibroplasia: Classification and objective evaluation
of incidence, natural history and resolution by fundus photography and
intravenous fluorescein angiography. Perspect Ophthalmol 2:175, 1978 35. Hindle NW: International classification of retrolental fibroplasia: A proposal. Can J Ophthalmol 17:107, 1982 36. Keith G: Retrolental fibroplasia: A new classification of the developing and cicatricial
changes. Aust J Ophthalmol 7:189, 1979 37. McCormick A: Retinopathy of prematurity. Curr Probl Pediatr 7:1, 1977 38. Patz A: Retrolental fibroplasia. Surv Ophthalmol 14:1, 1969 39. Schaffer D, Johnson L, Quinn G et al: A classification of retrolental fibroplasia to evaluate vitamin E therapy. Ophthalmology 86:1749, 1979 40. Uemura Y: Current status of retrolental fibroplasia: Report of the Joint committee
for the study of RLF. Jpn J Ophthalmol 21:366, 1977 41. The International Committee for the Classification of the Late Stages of
Retinopathy of Prematurity: An international classification of retinopathy
of prematurity: II. The classification of retinal detachment. Arch
Ophthalmol 105:906, 1987 42. Screening examination of premature infants for retinopathy of prematurity: A
joint statement of the American Academy of Pediatrics, the American
Association for Pediatric Ophthalmology and Strabismus, and the American
Academy of Ophthalmology. Ophthalmology 104:888, 1997 43. Fierson W, Palmer E, Biglan A et al: Retinopathy of prematurity guidelines [letter]. Ophthalmology 105:941, 1998 44. Wright K, Anderson M, Walker E et al: Should fewer premature infants be screened for retinopathy of prematurity
in the managed care era? Pediatrics 102:31, 1998 45. Report of a Working Party of the Royal College of Ophthalmologists and
the British Association of Perinatal Medicine. Early Hum Dev 46:239, 1996 46. Yamashita Y: Studies on retinopathy of prematurity: III. Cryocautery for retinopathy
of prematurity. Jpn J Ophthalmol 26:385, 1972 47. Majima A, Takahashi M, Hibino Y: Clinical observations of photocoagulation on retinopathy of prematurity. Jpn J Ophthalmol 30:93, 1976 48. Nagata M, Yamagishi N, Ikeda S: Summarized results of the treatment of acute proliferative retinopathy
of prematurity during the past 15 years at Tenri Hospital. Acta Soc Ophthalmol Jpn 86:1236, 1982 49. Sasaki K, Yamashita Y, Maekawa T et al: Treatment of retinopathy of prematurity in active stages by cryocautery. Jpn J Ophthalmol 20:384, 1976 50. Takagi I: Treatment of acute retrolental fibroplasia. Nippon Ganka Gakkai Zasshi 82:323, 1978 51. Ben-Sira I, Nissenkorn I, Grunwald E et al: Treatment of acute retrolental
fibroplasia by cryopexy. Br J Ophthalmol 64, 1980 52. Hindle N: Cryotherapy for retinopathy of prematurity to prevent retrolental fibroplasia. Can J Ophthalmol 17:207, 1982 53. Kingham J: Acute retrolental fibroplasia: Treatment by cryosurgery. Arch Ophthalmol 96:2049, 1978 54. Mousel D, Hoyt C: Cryotherapy for retinopathy of prematurity. Ophthalmology 87:1121, 1980 55. Mousel D: Cryotherapy for retinopathy of prematurity: A personal retrospective. Ophthalmology 92:375, 1985 56. Palmer E, Goodman S: A pilot randomized trial of cryotherapy for retinopathy of prematurity. Invest Ophthalmol Vis Sci 28(Suppl):105, 1987 57. Payne J, Patz A: Treatment of acute retrolental fibroplasia. Trans Am Acad Ophthalmol Otolaryngol 76:1234, 1972 58. Reisner S, Amir J, Shohat M et al: Retinopathy of prematurity: Incidence and treatment. Arch Dis Child 60:698, 1985 59. Tasman W: Multicenter trial of cryotherapy for retinopathy of prematurity. Arch Ophthalmol 106:463, 1988 60. Cryotherapy for Retinopathy of Prematurity Cooperative Group: Multicenter
trial of cryotherapy for retinopathy of prematurity: Preliminary results. Arch
Ophthalmol 106: 471, 1988 61. Cryotherapy for Retinopathy of Prematurity Cooperative Group: Multicenter
trial of cryotherapy for retinopathy of prematurity: 1-year outcome. Structure
and function. Arch Ophthalmol 108:1408, 1990 62. Cryotherapy for Retinopathy of Prematurity Cooperative Group: Multicenter
trial of cryotherapy for retinopathy of prematurity: 3-½ year
outcome. Structure and function. Arch Ophthalmol 111:339, 1993 63. Cryotherapy for Retinopathy of Prematurity Cooperative Group: Multicenter
trial of cryotherapy for retinopathy of prematurity: Snellen visual
acuity and structural outcome at 5 ½ years after randomization. Arch
Ophthalmol 114:417, 1996 64. Greven C, Tasman W: Rhegmatogenous retinal detachment following cryotherapy for retinopathy
of prematurity. Arch Ophthalmol 107:1017, 1989 64a. Quinn GE, Dobson V, Hardy RJ, et al: Visual fields measured with double-arc perimetry in eyes with threshold
retinopathy of prematurity from the cryotherapy for retinopathy of prematurity
trial. The CRYO-Retinopathy of Prematurity Cooperative Group. Ophthalmol 103:1432, 1996 65. McNamara J, Tasman W, Brown G et al: Laser photocoagulation for stage 3+ retinopathy of prematurity. Ophthalmology 98:576, 1991 66. McNamara J, Tasman W, Vander J et al: Diode laser photocoagulation for retinopathy of prematurity: Preliminary
results. Arch Ophthalmol 110:1714, 1992 67. Iverson D, Trese M, Orgel I et al: Laser photocoagulation for threshold retinopathy of prematurity. Arch Ophthalmol 108:1342, 1991 68. Landers M, Toth C, Semple H et al: Treatment of retinopathy of prematurity with argon laser photocoagulation. Arch Ophthalmol 110:44, 1992 69. Goggin M, O'Keefe M: Diode laser for retinopathy of prematurity: Early outcome. Br J Ophthalmol 77:559, 1993 70. Seiberth V, Linderkamp O, Vardarli I et al: Diode laser photocoagulation for stage 3+ retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 233:489, 1995 71. Hammer M, Pusateri T, Hess J et al: Threshold retinopathy of prematurity. Transition from cryopexy to laser
treatment. Retina 15:486, 1995 72. Noonan C, Clarke D: Trends in the management of stage 3 retinopathy of prematurity. Br J Ophthalmol 80:278, 1996 73. Fleming T, Runge P, Charles S: Diode laser photocoagulation for prethreshold, posterior retinopathy of
prematurity. Am J Ophthalmol 114:589, 1992 74. Capone AJ, Diaz-Rohena R, Sternberg PJ et al: Diode laser photocoagulation for zone I threshold retinopathy of prematurity. Am J Ophthalmol 116:444, 1993 75. Vander J, Handa J, McNamara J et al: Early treatment of posterior retinopathy of prematurity: A controlled trial. Ophthalmology 104:1731, 1997 76. Connolly B, McNamara J, Sharma S et al: A comparison of laser photocoagulation with trans-scleral cryotherapy in
the treatment of threshold retinopathy of prematurity. Ophthalmology 105:1628, 1998 77. White J, Repka M: Randomized comparison of diode laser photocoagulation versus cryotherapy
for threshold retinopathy of prematurity: 3-year outcome. J Pediatr Ophthalmol Strabismus 34:83, 1997 78. Laws F, Laws D, Clark D: Cryotherapy and laser treatment for acute retinopathy of prematurity: Refractive
outcomes. A longitudinal study. Br J Ophthalmol 81:12, 1997 79. Knight-Nanan D, O'Keefe M: Refractive outcome in eyes with retinopathy of prematurity treated with
cryotherapy or diode laser: 3-year follow up. Br J Ophthalmol 80:998, 1996 80. Algawi A, Goggin M, O'Keefe M: Refractive outcome following diode laser versus cryotherapy for eyes with
retinopathy of prematurity. Br J Ophthalmol 78:612, 1994 81. Drack A, Burke J, Pulido J et al: Lenticular opacities as a complication of argon laser photocoablation in
an infant with retinopathy of prematurity. Am J Ophthalmol 113: 583, 1992 82. Christiansen S, Bradford J: Cataract in infants treated with argon laser photocoagulation for threshold
retinopathy of prematurity. Am J Ophthalmol 120:264, 1995 83. Pogrebniak A, Bolling J, Stewart M: Argon laser-induced cataract in an infant with retinopathy of prematurity [letter]. Am J Ophthalmol 117:261, 1994 84. Campolattaro B, Lueder T: Cataract in infants treated with argon laser photocoagulation for threshold
retinopathy of prematurity. Am J Ophthalmol 119:175, 1995 85. Capone AJ, Drack A: Transient lens changes after diode laser photocoablation for retinopathy
of prematurity [letter]. Am J Ophthalmol 118:533, 1994 86. Simons B, Wilson M, Hertle R et al: Bilateral hyphemas and cataracts after diode laser retinal photoablation
for retinopathy of prematurity. J Pediatr Ophthalmol Strabismus 35:185, 1998 87. Tasman W: Retinal detachment in retinopathy of prematurity. In Silverman
W, Flynn J (eds): Contemporary Issues in Fetal and Neonatal Medicine: Retinopathy
of Prematurity, p 232. Boston: Blackwell Scientific Publications, 1985 88. McPherson A, Mintz-Hittner H, Lemos R: Retinal detachment in young premature infants with acute retrolental fibroplasia: Thirty-two
new cases. Ophthalmology 89:160, 1982 89. Topilow H, Ackerman A, Wang F: The treatment of advanced retinopathy of prematurity by cryotherapy and
scleral buckling surgery. Ophthalmology 92:379, 1985 90. Greven C, Tasman W: Scleral buckling in stages 4B and 5 retinopathy of prematurity. Ophthalmology 97:817, 1990 91. Wicharz A, Paulmann H, Stojanov D: Ergebnisse der operativen therapie forgeschrittener Stadien der retinopathia
prämaturorum. Fortschr Ophthalmol 88:477, 1991 92. Noorily S, Small K, de Juan E et al: Scleral buckling surgery for stage 4B
retinopathy of prematurity. Ophthalmology 1992; 99:263 93. Trese M: Scleral buckling for retinopathy of prematurity. Ophthalmology 101:23, 1994 94. Ricci B, Santo A, Ricci F et al: Scleral buckling surgery in stage 4 retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol 234(Suppl):S38, 1996 95. Sucheski B, Tasman W, Vander J et al: Visual outcomes of scleral buckling
for stage 4B and 5 retinopathy of prematurity. Ophthalmology (submitted
for publication) 96. Schepens C: Clinical and research aspects of subtotal open-sky vitrectomy. Am J Ophthalmol 91:143, 1981 97. Hirose T, Schepens C: Open-sky vitrectomy in severe retinal detachment caused by advanced retinopathy
of prematurity. Ophthalmology 91(Suppl):32, 1984 98. Tasman W, Borrone R, Bolling J: Open-sky vitrectomy for total retinal detachment in retinopathy of prematurity. Ophthalmology 94:449, 1987 99. Hirose T, Katsumi O, Mehta M et al: Vision in stage 5 retinopathy of prematurity after retinal reattachment
by open-sky vitrectomy. Arch Ophthalmol 111:345, 1993 100. Charles S: Retinopathy of Prematurity, p 158. Baltimore: Williams & Wilkins, 1987 101. de Juan E Jr, Machemer R: Retinopathy of prematurity: Surgical technique. Retina 7:63, 1987 102. Choi W, Yu Y: Surgical management for stage 5 retinopathy of prematurity. Korean J Ophthalmol 8:37, 1994 103. Mintz-Hittner H, O'Malley R, Kretzer F: Long-term form idedtification vision after early, closed, lensectomy-vitrectomy
for stage 5 retinopathy of prematurity. Ophthalmology 104:454, 1997 104. Trese M, Droste P: Long-term postoperative results of a consecutive series of stages 4 and 5 retinopathy
of prematurity. Ophthalmology 105:992, 1998 105. Charles S: Vitrectomy for stage 5 retinopathy of prematurity. American
Academy of Ophthalmology Annual Meeting, New Orleans, 1989 106. Maguire A, Trese M: Lens-sparing vitreoretinal surgery in infants. Arch Ophthalmol 110:284, 1987 107. McNamara J: Retinopathy of prematurity. In Tasman W (ed): Clinial Decisions
in Medical Retinal Diseases, p 224. Philadelphia: Mosby, 1996 108. Seaber J, Machemer R, Eliott D et al: Long-term visual results of children after initially successful vitrectomy
for stage V retinopathy of prematurity. Ophthalmology 102: 199, 1995 109. Fuchino Y, Hayashi H, Kono T et al: Long-term follow-up of visual acuity in eyes with stage 5 retinopathy of
prematurity after closed vitrectomy. Am J Ophthalmol 120:308, 1995 110. Quinn G, Dobson V, Barr C et al: Visual acuity of eyes after vitrectomy for ROP: Follow-up at 5-½ years. Ophthalmology 103:595, 1996 111. Knight-Nanan D, Algawi K, Bowell R et al: Advanced cicatricial retinopathy of prematurity: Outcome and complications. Br J Ophthalmol 80:343, 1996 112. Choi W, Yu Y: Antomical and visual results of vitreous surgery for advanced retinopathy
of prematurity. Korean J Ophthalmol 12:60, 1998 113. Faris B, Tolentino F, Freeman H et al: Retrolental fibroplasia in the cicatricial stage: Fundus and vitreous findings. Arch Ophthalmol 1971:661, 1971 114. Nissenkorn I, Yassur Y, Mashkowski D et al: Myopia in premature babies with and without retinopathy of prematurity. Br J Ophthalmol 67:170, 1983 115. Tasman W: Late complications of retrolental fibroplasia. Trans Am Acad Ophthalmol Otolaryngol 86:1724, 1979 116. Tasman W: Retinal detachment in retrolental fibroplasia. Graefes Arch Clin Exp Ophthalmol 195:129, 1975 117. Tasman W: Vitreoretinal changes in cicatricial retrolental fibroplasia. Trans Am Ophthalmol Soc 68:548, 1970 118. Blodi F: Symposium: Retrolental fibroplasia (retinopathy of prematurity): Management. Trans Am Acad Ophthalmol Otolaryngol 59:367, 1955 119. Kushner B, Sondheimer S: Medical management of glaucome associated with cicatricial retinopathy
of prematurity. Am J Ophthalmol 94:313, 1982 120. Pollard Z: Lensectomy for secondary angle-closure glaucoma in advanced cicatricial
retrolental fibroplasia. Ophthalmology 91:395, 1984 121. Smith J, Shivitz I: Angle-closure glaucoma in adults with cicatricial retinopathy of prematurity (ROP). Arch Ophthalmol 102:371, 1984 122. Tasman W, Brown C: Progressive visual loss in adults with retinopathy of prematurity (ROP). Trans Am Ophthalmol Soc 86:367, 1988 123. Irvine A: Progressive visual loss in adults with retinopathy of prematurity (ROP). Trans Am Ophthalmol Soc 86:375, 1988 124. Benson W: Familial exudative vitreoretinopathy. Trans Am Ophthalmol Soc 93:473, 1995 |