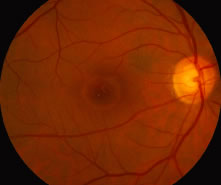
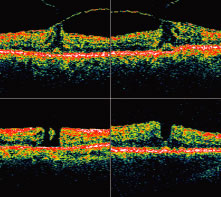
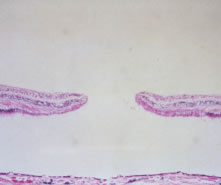
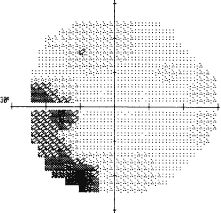1. Knapp. Uber isolierite zerreissungen der aderhaut in folge von traumen
auf dem augapfel. Arch Aug Ohrenheilk 1869;1:6 2. Fine, S. Discussion, Macular Holes. Ophthalmology 1993;100:871 3. McDonnell PJ, Fine SL, Hillis AI. Clinical features of idiopathic macular
cysts and holes. Am J Ophthalmol 1982;93:777 4. Eye Disease Case-Control Study Group. Risk factors for idiopathic macular
holes. Am J Ophthalmol 1994;118:754 5. Morgan CM, Schatz H. Involutional macular thinning. A pre-macular hole
condition. Ophthalmology 1986;93:153 6. Aaberg TM. Macular holes: A review. Surv Ophthalmol 1970;15:139 7. Noyes HD. Detachment of the retina, with laceration at the macula lutea. Trans
Am Ophthal Soc 1871;1:128 8. Fuchs E. Zur Veranderung der Macula Lutea Nach Contusion. Ztschr Augenheilk 1901;6:181 9. Coats G. The pathology of macular holes. Roy Lond Hosp Rep 1907;17:69 10. Kuhnt H. Uber Eine Eigenthumliche Veranderung der Netzhaut ad Maculam (Retinitis
Atrophicans Sive Rareficans Centralis). Ztschr Augenheilk 1900;3:105 11. Gifford SR. An evaluation of ocular angiospasm. Trans Am Ophthalmol Soc 1943;48:19 12. Lister W. Holes in the retina and their clinical significance. Br J Ophthalmol 1924;8:1 13. Gass JDM. Idiopathic senile macular hole: Its early stages and pathogenesis. Arch
Ophthalmol 1988;106:629 14. Gass JDM. Reappraisal of biomicroscopic classification of stages of development
of a macular hole. Am J Ophthalmol 1995;119:752 15. Kishi S, Takahashi H. Three-dimensional observations of developing macular
holes. Am J Ophthalmol 2000;130:65 16. Haouchine B, Massin P, Gaudric A. foveal pseudocyst as the first step in
macular hole formation. Ophthalmology 2001;108:15 17. Guyer DR, Green WR, de Bustrus S et al. Histopathologic features of idiopathic
macular holes and cysts. Ophthalmology 1990;97:1045 18. Frangieh GT, Green WR, Engel HM. A histopathologic study of macular cysts
and holes. Retina 1981;1:311 19. Gass JD. Lamellar macular hole: A complication of cystoid macular edema
after cataract extraction. Arch Ophthalmol 1976;94:793 20. Ho AC, Guyer DR, Fine SL. Macular hole. Surv Ophthalmol 1998;42:393 21. Watzke RC, Allen L. Subjective slitbeam sign for macular disease. Am J
Ophthalmol 1969;68:449 22. Martinez J, Smiddy WE, Kim J et al. Differentiating macular holes from
macular pseudoholes. Am J Ophthalmol 1994;117:762 23. Hee MR, Puliafito CA, Wong C et al. Optical coherence tomography of macular
holes. Ophthalmology 1995;102:748 24. Puliafito CA, Hee MR, Lin CP et al. Imaging of macular diseases with optical
coherence tomography. Ophthalmology 1995;102:217 25. Gallemore RP, Jumper JM, McCuen BW II et al. Diagnosis of vitreoretinal
adhesions in macular disease with optical coherence tomography. Retina 2000;20:115 26. Smith RG, Hardman LSJ, Galloway NR. Visual performance in idiopathic macular
holes. Eye 1990;4:190 27. Thompson JT, Hiner CJ, Glaser BM et al. Fluorescein angiographic characteristics
of macular holes before and after vitrectomy with transforming
growth factor beta-2. Am J Ophthalmol 1994;117:291 28. Klein BR, Hiner CJ, Glaser BM et al. Fundus photographic and fluorescein
angiographic characteristics of pseudoholes of the macula in eyes with
epiretinal membranes. Ophthalmology 1995;102:768 29. Allen AW, Gass JD. Contraction of a perifoveal epiretinal membrane simulating
a macular hole. Am J Ophthalmol 1976;82:684 30. Gass JD, Joondeph BC. Observations concerning patients with suspected impending
macular holes. Am J Ophthalmol 1990;109:638 31. Guyer DR, Green WR. Idiopathic macular holes and precursor lesions. Proceedings
of the Symposium on Retina and Vitreous, New Orleans Academy
of Ophthalmology. New York, Kugler Publications, 1993:135–162. 32. Klein BR, Hiner CJ, Glaser BM et al. Fundus photographic and fluorescein
angiographic characteristics of pseudoholes of the macula in eyes with
epiretinal membranes. Ophthalmology 1995;102:768 33. Slezak H. On findings resembling holes and cysts of the posterior pole. Graefes
Arch Klin Exp Ophthalmol 1967;173:168 34. Fish RH, Arnand R, Izbrand DJ. Macular pseudoholes. Clinical features and
accuracy of diagnosis. Ophthalmology 1992;99:1665 35. Gass JD. Lamellar macular hole: A complication of cystoid macular edema
after cataract extraction. Arch Ophthalmol 1976;94:793 36. Patel B, Duvall J, Tullo AB. Lamellar macular hole associated with idiopathic
juxtafoveolar telangiectasia. Br J Ophthalmol 1988;72:550 37. Smiddy WE, Gass JD. Masquerades of macular holes. Ophthalmic Surg 1995;26:16 38. Gass JD, VanNewkirk M. Xanthic scotoma and yellow foveolar shadow caused
by a pseudo-operculum after vitreofoveal separation. Retina 1992;12:242 39. Gross JG, Freeman WR. Posttraumatic yellow maculopathy. Retina 1990;10:37 40. de Bustros S. Vitrectomy for prevention of macular holes. Results of a
randomized multicenter clinical trial. Vitrectomy for Prevention of Macular
Hole Study Group. Ophthalmology 1994;101:1055 41. Kokame GT, de Bustros S. Visual acuity as a prognostic indicator in stage 1 macular
holes. The Vitrectomy for Prevention of Macular Hole Study
Group. Am J Ophthalmol 1995;120:112 42. Guyer DR, de Bustros S, Diener WM et al. Observations on patients with
idiopathic macular holes and cysts. Arch Ophthalmol 1992;110:1264 43. Hikichi T, Yoshida A, Akiba J et al. Prognosis of stage 2 macular holes. Am
J Ophthalmol 1995;119:571 44. James M, Feman SS. Macular holes. Graefes Arch Clin Exp Ophthalmol 1980;215:59 45. Hikichi T, Yoshida A, Akiba J et al. Natural outcomes of stage 1, 2, 3, and 4 idiopathic
macular holes. Br J Ophthalmol 1995;79:517 46. Aaberg TM. Macular holes. Am J Ophthalmol 1970;69:555 47. Mikuni M, Kobayashi S, Yaoeda H. Treatment of retinal detachment with macular
hole. Nippon Ganka Kiyo 1967;18:659 48. Yaoeda H. Clinical observation on macular hole. Nippon Ganka Gakkai Zasshi 1967;71:1723 49. Bidwell AE, Jampol LM. Macular holes and excellent visual acuity. Arch
Ophthalmol 1988;106:1350 50. Lewis H, Cowan GM, Straatsma BR. Apparent disappearance of a macular hole
associated with development of an epiretinal membrane. Am J Ophthalmol 1986;102:172 51. Sjaarda RN, Frank DA, Glaser BM et al. Resolution of an absolute scotoma
and improvement of relative scotomata after successful macular hole
surgery. Am J Ophthalmol 1993;116:129 52. Akiba J, Kakehashi A, Arzabe CW et al. Fellow eyes in idiopathic macular
hole cases. Ophthalmic Surg 1992;23:594 53. Bronstein MA, Trempe CL, Freeman HM. Fellow eyes with macular holes. Am
J Ophthalmol 1981;92:757 54. Ezra E, Wells JA, Gray RH et al. Incidence of idiopathic full-thickness
macular holes in fellow eyes: A 5-year prospective natural history study. Ophthalmology 1998;105:353 55. Benson WE, Cruickshanks KC, Fong DS et al. Surgical management of macular
holes. A report by the American Academy of Ophthalmology. Ophthalmology 2001;108:1328 56. Kim JW, Freeman WR, Azen SP et al. Prospective randomized trial of vitrectomy
or observation for stage 2 macular holes. Am J Ophthalmol 1996;121:605 57. Freeman WR, Azen SP, Kim JW et al. Vitrectomy for the treatment of full-thickness
stage 3 or 4 macular holes. Results of a multicentered randomized
clinical trial. Arch Ophthalmol 1997;115:11 58. Kelly NE, Wendel RT. Vitreous surgery for idiopathic macular holes. Results
of a pilot study. Arch Ophthalmol 1991;109:654 59. Thompson JT, Smiddy WE, Glaser BM et al. Intraocular tamponade duration
and success of macular hole surgery. Retina 1996;16:373 60. Tornambe PE, Poliner LS, Grote K. Macular hole surgery without face-down
positioning. A pilot study. Retina 1997;17:179 61. Liggett PE, Skolik DS, Horio B et al. Human autologous serum for the treatment
of full-thickness macular holes. A preliminary study. Ophthalmology 1995;102:1071 62. Gaudric A, Massin P, Paques M et al. Autologous platelet concentrate for
the treatment of full-thickness macular holes. Graefes Arch Clin Exp
Ophthalmol 1995;233:549 63. Olsen TW, Sternberg P Jr, Capone A Jr et al. Macular hole surgery using
thrombin-activated fibrinogen and selective removal of the internal limiting
membrane. Retina 1998;18:322 64. Vine AK, Johnson MW. Thrombin in the management of full-thickness macular
holes. Retina 1996;16:474 65. Glaser BM, Michels RG, Kuppermann BD et al. Transforming growth factor
beta-2 for the treatment of full-thickness macular holes: A prospective
and randomized study. Ophthalmology 1992;99:1162 66. Tilanus MA, Deutman AE. Full-thickness macular holes treated with vitrectomy
and tissue glue. Int Ophthalmol 1994-1995;18:355 67. Smiddy WE, Glaser BM, Thompson JT et al. Transforming growth factor-beta 2 significantly
enhances the ability to flatten the rim of the subretinal
fluid surrounding macular holes. Preliminary anatomic results of
a multicenter prospective randomized study. Retina 1993;13:296 68. Thompson JT, Smiddy WE, Williams GA et al. Comparison of recombinant transforming
growth factor-beta-2 and placebo as an adjunctive agent for
macular hole surgery. Ophthalmology 1998;105:700 69. Paques M, Chastang C, Mathis A et al. Effect of autologous platelet concentrate
in surgery for idiopathic macular hole: Results of a multicenter, double-masked, randomized trial. Platelets in Macular Hole Surgery
Group. Ophthalmology 1999;106:932 70. Park DW, Sipperley JO, Sneed SR et al. Macular hole surgery with internal-limiting
membrane peeling and intravitreal air. Ophthalmology 1999;106:1392 71. Brooks HL Jr. Macular hole surgery with and without internal limiting membrane
peeling. Ophthalmology 2000;107:1939 72. Margherio RR, Margherio AR, Williams GA et al. Effect of perifoveal tissue
dissection in the management of acute idiopathic full-thickness macular
holes. Arch Ophthalmol 2000;118:495 73. Gandorfer A, Haritoglou C, Gass C et al. Indocyanine green-assisted peeling
of the internal limiting membrane may cause retinal damage. Am J
Ophthalmol 2001;132:431 74. Sippy BD, Engelbrecht NE, Hubbard GB. Indocyanine green effect on cultured
human retinal pigment epithelial cells: Implications for macular hole
surgery. Am J Ophthalmol 2001;132:433 75. Smiddy WE, Feuer W, Cordahi G. Internal limiting membrane peeling in macular
hole surgery. Ophthalmology 2001;108:1471 76. Sjaarda RN, Glaser BM, Thompson JT et al. Distribution of iatrogenic retinal
breaks in macular hole surgery. Ophthalmology 1995;102:1387 77. Park SS, Marcus DM, Duker JS et al. Posterior segment complications after
vitrectomy for macular hole. Ophthalmology 1995;102:775 78. Banker AS, Freeman WR, Kim JW et al. Vision-threatening complications of
surgery for full-thickness macular holes. Vitrectomy for Macular Hole
Study Group. Ophthalmology 1997;104:1442 79. Thompson JT, Glaser BM, Sjaarda RN et al. Progression of nuclear sclerosis
and long-term visual results of vitrectomy with transforming growth
factor beta-2 for macular holes. Am J Ophthalmol 1995;119:819 80. Duker JS, Wendel R, Patel AC et al. Late re-opening of macular holes after
initially successful treatment with vitreous surgery. Ophthalmology 1994;101:1373 81. Paques M, Massin P, Blain P et al. Long-term incidence of reopening of
macular holes. Ophthalmology 2000;107:760 82. Christmas NJ, Smiddy WE, Flynn HW Jr. Reopening of macular holes after
initially successful repair. Ophthalmology 1998;105:1835 83. Poliner LS, Tornambe PE. Retinal pigment epitheliopathy after macular hole
surgery. Ophthalmology 1993;99:1671 84. Duker JS. Retinal pigment epitheliopathy after macular hole surgery [Letter]. Ophthalmology 1993;100:1604 85. Akduman L, Del Priore LV, Kaplan HJ. Exudative retinal detachment as a
complication of macular hole surgery. Ophthalmology 1996;103(Suppl):124. 86. Chen CJ. Glaucoma after macular hole surgery. Ophthalmology 1998;105:94 87. Coll GE, Chang S, Lewis H. Macular hole surgery and proliferative vitreoretinopathy. Ophthalmology 1996;103(Suppl):125 88. Welch JC. Dehydration injury as a possible cause of visual field defect
after pars plana vitrectomy for macular hole. Am J Ophthalmol 1997;124:698 89. Ohji M, Nao-I N, Saito Y et al. Prevention of visual field defect after
macular hole surgery by passing air used for fluid-air exchange through
water. Am J Ophthalmol 1999;127:62 |