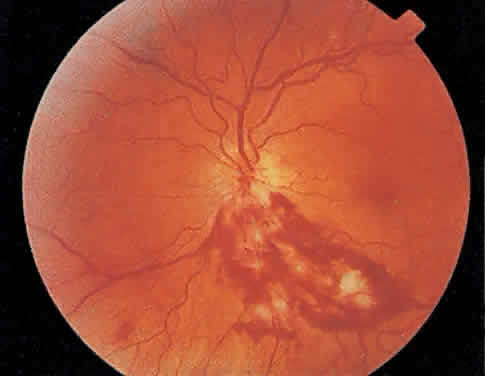
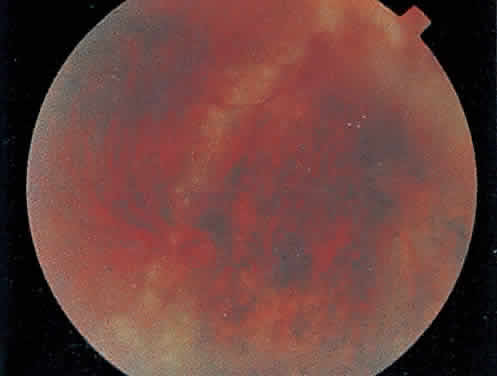
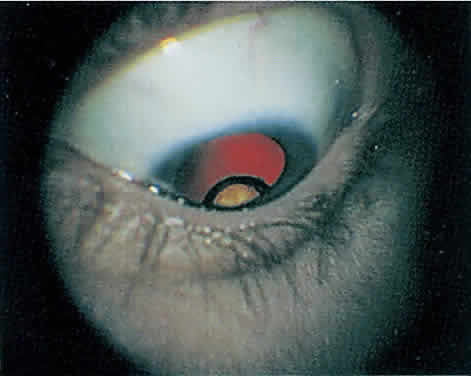
The hallmark lesion of CMV retinitis is a necrotizing, full-thickness retinitis that results in retinal cell destruction. CMV often initially affects retinal tissue adjacent to major retinal blood vessels or the optic disc (Fig. 1). This is consistent with the concept that the virus is spread to the retina hematogenously. In most cases, the pattern of infection is classic and distinctive, making clinical diagnosis straightforward. The area of active retinitis has a granular, dirty-white appearance. As the virus attacks the endothelial cells of blood vessels, hemorrhage is common. Advancement in the retinitis by both direct cell-to-cell transmission as well as spread by way of adjacent satellite lesions can be seen. Except for cases in which retinitis is acute, it is common to see areas of healed retinitis beside areas of active necrosis. Areas of burned-out necrosis show absence of any retinal tissue, whereas the underlying retinal pigment epithelium assumes a “salt and pepper” appearance. CMV retinitis can present initially as either large areas of retinal necrosis with hemorrhage or one or more small, focal areas of retinal whitening.1,12,14 These small, focal lesions may on occasion be confused with cotton-wool spots or lesions of toxoplasmosis.5,27 Unlike cotton-wool spots, focal areas of CMV may appear outside the posterior pole. These early, focal infiltrates of CMV may not be associated with retinal hemorrhages or vitreous cells.
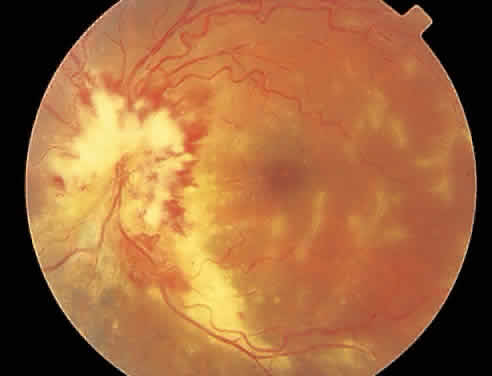
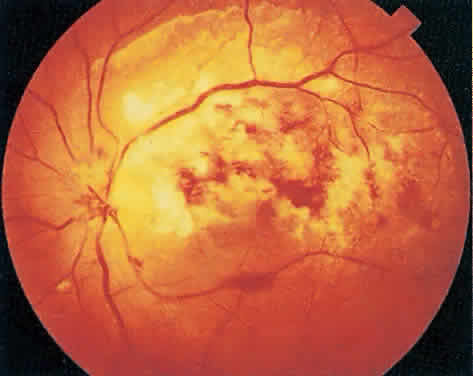
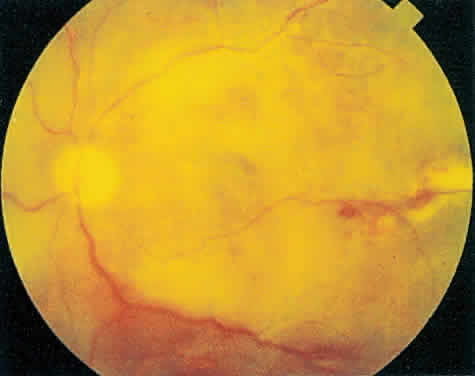
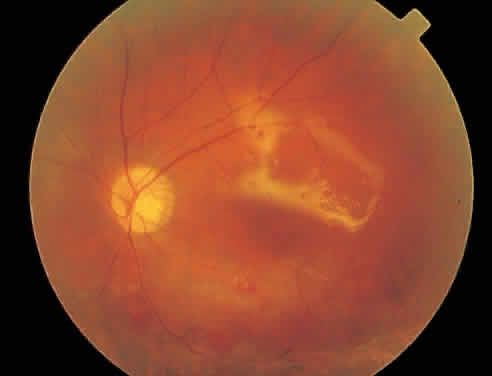
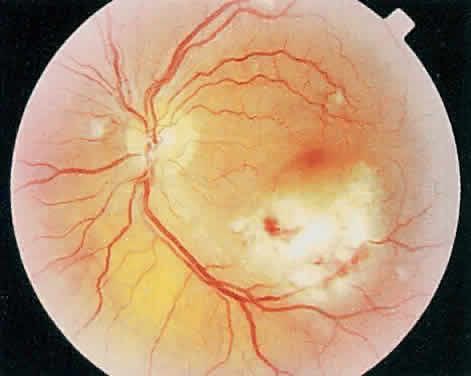
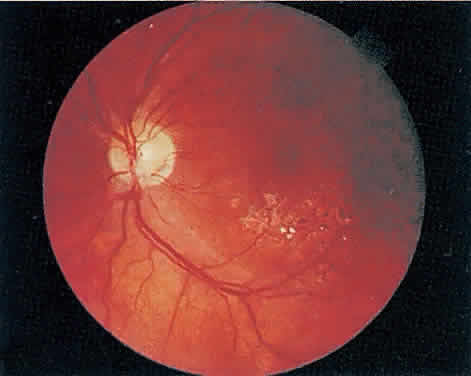
Over a course that usually spans weeks, infiltrates of CMV tend to assume two different patterns of clinical disease.11,12 The first pattern is called hemorrhagic and is characterized by broad geographic zones of retinal whitening. These large, geographic lesions are usually in close proximity to a major retinal blood vessel or the optic nerve. Satellite lesions are common. When the retinal necrosis associated with CMV retinitis becomes widespread, it is almost invariably associated with retinal hemorrhages. Although the border between necrotic and unaffected retina is sharply demarcated, the border itself appears irregular and jagged. Exudation into the retina or subretinal space may be seen, adding to the granular appearance of the retinitis. Juxtaposition of large zones of white, granular necrosis with those of red retinal hemorrhage has led this appearance of CMV retinitis to be described as either “pizza-pie” or “cheese and ketchup.” The retinal blood vessels, both arteries and veins, in the areas of necrosis commonly appear sheathed, secondary to a vasculitis. As a consequence, secondary retinal vascular occlusions, especially branch retinal vein obstructions, may occur in the course of CMV retinitis. Immune-mediated vascular damage may play a role in the vasculitis.12 Central healing of these lesions will occur as the infection progresses. Avasculitis resembling “frosted branch angiitis” hasbeen reported (Fig. 2).28 A second pattern of CMVretinitis has been labeled “granular” or “brushfireborder.” In this appearance, the focal granular infiltrates enlarge slowly across a line, leaving ever-increasing areas of destroyed retina and atrophic retinal pigment epithelium behind. Hemorrhages and vitreous cells are a less prominent feature. There appears to be direct cell-to-cell transfer of infected virions in this pattern of infection (Figs. 3 and 4). The brushfire border is commonly seen in CMV retinitis lesions anterior to the equator (Fig. 5). The significance, if any, of these two clinical patterns of CMV retinitis is not known, and, in some eyes, both patterns of disease can be seen simultaneously or in sequence. Progression of retinitis has been defined in clinical trials as movement of a lesion border at least 750 μm along a front that is 750 μm or more in length, development of a new CMV lesion in a previously involved eye or in the uninvolved fellow eye of a patient with baseline unilateral disease.29 Without treatment or improvement in the host's immune system, CMV retinitis is a relentless, slowly progressive infection resulting in blindness caused by total retinal necrosis, retinal detachment, or optic nerve involvement, in any combination.
|
|
|
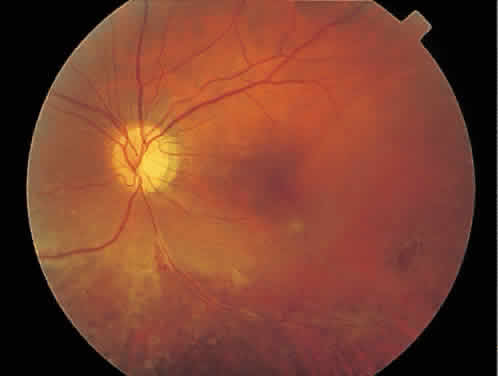
CMV infection can affect the optic nerve either directly or by extension from adjacent retinitis (see Fig. 2).30–32 When direct involvement occurs, optic neuritis with profound, irreversible visual loss usually develops. Several cases of CMV optic neuritis associated with adjacent retinitis have been treated successfully. Exudative retinal detachment can occur during the course of CMV retinitis as well.4,12,30 The subretinal fluid is seen primarily inferiorly in the fundus and shifts with position. No retinal break will be evident; however, in areas of extremely thin, atrophic retina, it can be quite difficult to determine whether a full-thickness defect is present. Exudative retinal detachment associated with CMV is usually nonprogressive and may respond to ganciclovir therapy.
Other features may include a mild to moderate anterior chamber cell and flare reaction. A hypopyon has rarely been reported in a renal transplant patient.4 Although mild vitreous cells are almost universally present, a florid vitritis severe enough to result in media opacity rarely develops from isolated CMV retinitis. In one patient who was immunosuppressed as a result of systemic corticosteroid therapy, a severe panuveitis associated with CMV retinitis was reported.32 More recently, an entity called immune recovery retinitis has been described.26,27 Immune-recovery uveitis is a chronic inflammatory syndrome associated with clinical immune reconstitution in AIDS patients with CMV retinitis who are taking HAART.33–35 It has emerged as an important cause of visual morbidity. Although immune recovery associated with HAART has allowed some patients to discontinue specific anti-CMV therapy, the rejuvenated immune response can be associated with this sight-threatening inflammation in some patients with preexisting CMV retinitis. Ocular features of immune-recovery uveitis include a significant vitritis that is more pronounced than that occurring with primary CMV retinitis. Additional features may include optic disc and macular edema. The mean CD4+ T-lymphocyte count in one study was 393 cells/mm3 at the time of diagnosis.33 Long-term vision-threatening complications related to this persistent inflammation include proliferative vitreoretinopathy, epiretinal membrane formation, posterior subcapsular cataracts, and severe postoperative inflammation.36
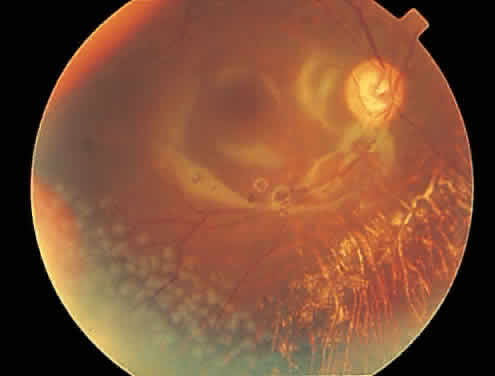
Rhegmatogenous retinal detachment occurs in 10% to 20% of eyes with CMV retinitis.37–39 However, before the AIDS epidemic, there were only five reported cases of this association.10,40 In patients living more than 1 year with CMV retinitis, risk of retinal detachment may be as high as 50%,41 which increases if more than 25% of peripheral retina is involved by disease.41,42 Retinal breaks in eyes with CMV retinitis typically occur within or at the border of necrotic atrophic retina (Fig. 6).43 The ensuing retinal detachments are typically difficult to repair with standard scleral buckling procedures. This is because of the location and number of retinal breaks, the difficulty in visualizing all breaks in necrotic retina, and the high incidence of associated proliferative vitreoretinopathy. In many cases, pars plana vitrectomy and retinal tamponade with silicone oil or long-acting intraocular gas is indicated (Figs. 7 and 8).37,44,45 However, scleral buckling may be considered in small peripheral retinal detachments when the entire involved area can be completely placed on the element. Laser photocoagulation demarcation has also been described to delimit macula-sparing CMV-related retinal detachment.46 Although anatomic success of macular reattachment with surgery is high, the visual results are often limited by the underlying disease process.47 When considering surgical repair of CMV-induced retinal detachment, consideration should be given to the potential for ambulatory vision, the patient's systemic condition and the status of the fellow eye.
|
|