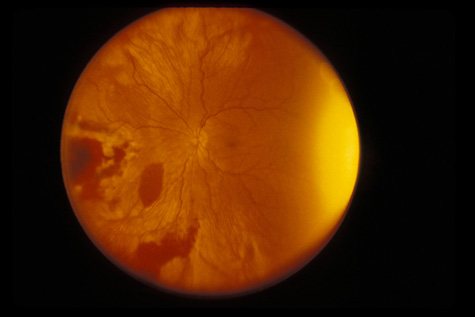
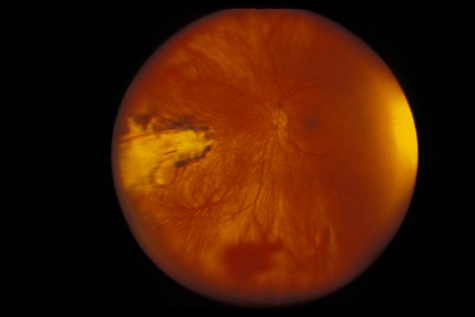
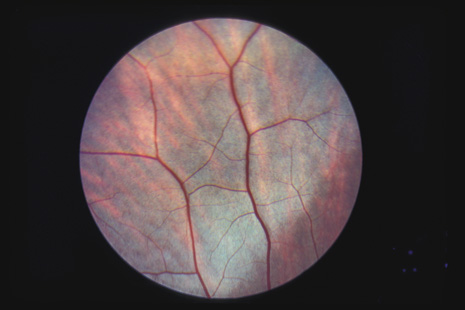
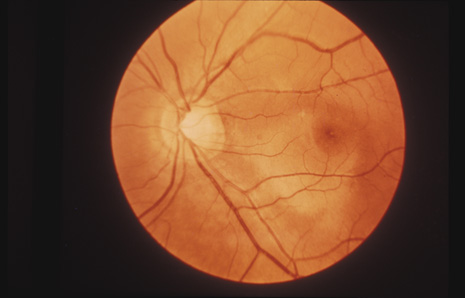
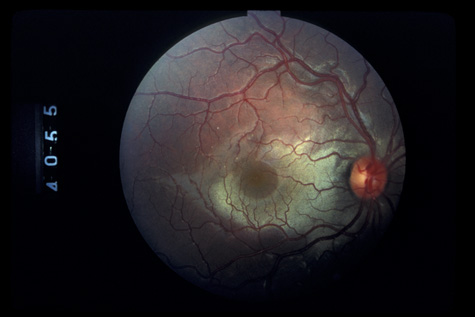
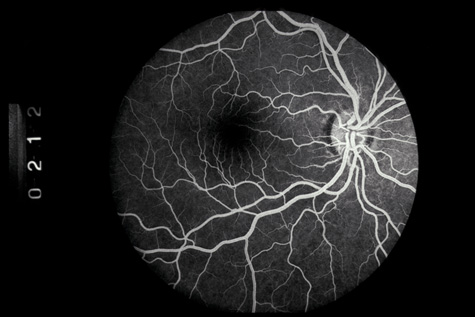
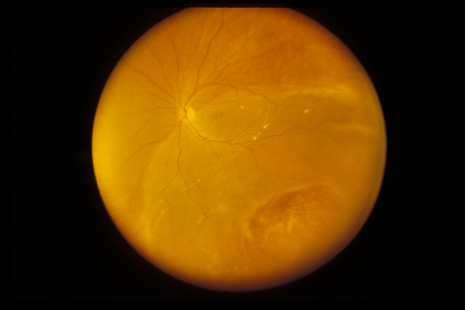
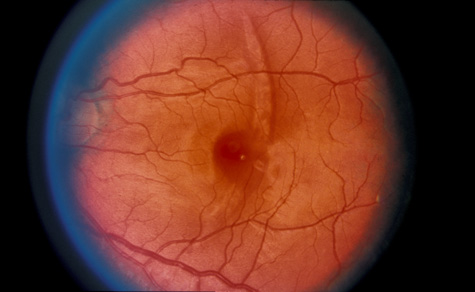
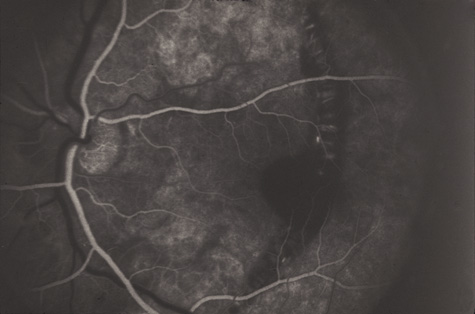
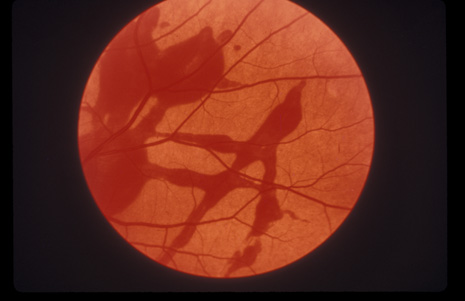
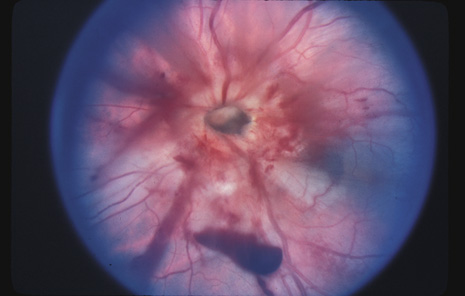
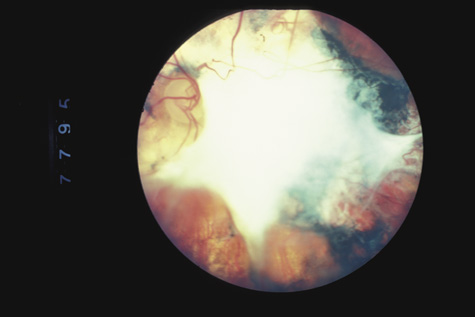
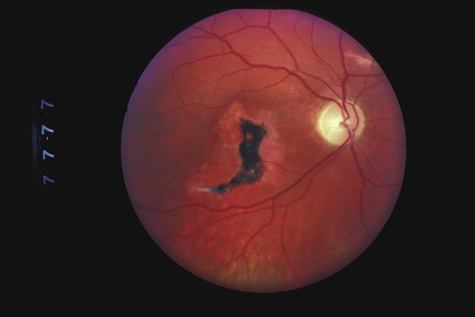
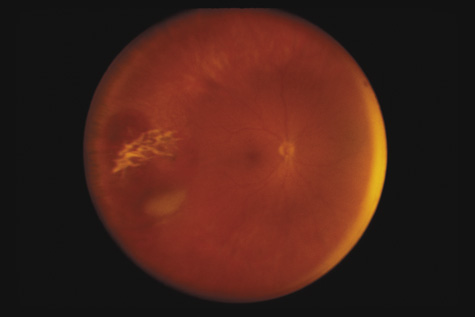
1. May DR, Kuhn FP, Morris RE, et al: The epidemiology of serious eye injuries from the United States Injury
Registry. Graefes Arch Clin Exp Ophthalmol 238:53, 2000 2. MacEwen CJ, Baines PS, Desai P: Eye injuries in children: the current picture. Br J Ophthalmol 83:933, 1999 3. Horn E, McDonald HR, Johnson RN: Soccer ball-related retinal injuries. Retina 20:604, 2000 4. Hollander DA, Aldave AJ. Ocular bungee cord injuries. Curr Opin Ophthalmol 13:167, 2002 5. Da Pozzo S, Pensiero S, Perissutti P: Ocular injuries by elastic cords in children. Pediatrics 106:E65, 2000 6. Thach AB, Ward TP, Hollifield RD, et al: Ocular injuries from paintball pellets. Ophthalmology 106:533, 1999 7. Fineman M, Fischer DH, Jeffers JB, et al: Changing trends in paintball sport-related ocular injuries. Arch Ophthalmol 118:60, 2000 8. Pearlman JA, Au Eong KG, Kuhn F, et al: Airbags and eye injuries: epidemiology, spectrum of injury, and analysis
of risk factors. Surv Ophthalmol 46:234, 2001 9. Stein J, Jaeger EA, Jeffers JB: Air bags and ocular injuries. Trans Am Ophth Soc 97:59, 1999 10. De Brito D, Challoner KR, Sehgal A, et al: The injury pattern of a new law enforcement weapon: the police bean bag. Ann Emerg Med 38:383, 2001 11. Endo S, Ishida N, Yamaguchi T: Tear in the trabecular meshwork caused by an airsoft gun. Am J Ophthalmol 131:656, 2001 12. Bullock JD, Ballal DR, Johnson DA: Ocular and orbital trauma from water balloon slingshots. Ophthalmology 104:878, 1997 13. Liggett PE, Pince KJ, Barlow W, et al: Ocular trauma in an urban population. Review of 1132 cases. Ophthalmology 97:581,1990 14. Katz J, Tielsch JM: Lifetime prevalence of ocular injuries from the Baltimore Eye Survey. Arch Ophthalmol 111:1564, 1993 15. Rodriguez JO, Lavina AM, Agarwal A: Prevention and treatment of common eye injuries in sports. Am Fam Physician 67:1481, 2003 16. Fong LP, Taouk Y: The role of eye protection in work-related eye injuries. Aust NZ J Ophthalmol 23:101, 1995 17. Farber AS: Preventing eye injuries: what to tell patients. Postgrad Med 89: 121, 1991 18. Giovinazzo VJ, Yannuzzi LA, Sorenson JA, et al: The ocular complications of boxing. Ophthalmology 94:587, 1987 19. Maguire JI, Benson WE: Retinal injury and detachment in boxers. JAMA 155:2451, 1986 20. Easterbrook M. Ocular injuries in racquet sports. Int Ophthalmol Clin 28:232, 1988 21. Knorr HL, Jonas JB: Retinal detachments by squash ball accidents. Am J Ophthalmol 122:260, 1996 22. Pashby TJ: Eye injuries in Canadian sports and recreational activities. Can J Ophthalmol 27:226, 1992 23. Joseph E, Zak R, Smith S, et al: Predictors of blinding or serious eye injury in blunt trauma. J Trauma 33:19, 1992 24. Courville CB: Coup-contrecoup mechanism of cranio-cerebral injuries. Arch Surg 45:19, 1942 25. Courville CB: Forensic neuropathology. J Forensic Sci 7:1, 1962 26. Wolter JR. Coup-contrecoup mechanism of ocular injuries. Am J Ophthalmol 56:785, 1963 27. Delori FO, Pomerantzeff O, Cox MS: Deformation of the globe under high-speed impact: its relation to contusion
injuries. Invest Ophthalmol 8:290, 1969 28. Bourne WB, McCarey B, Kaufman H: Clinical specular microscopy. Trans Am Acad Ophthalmol Otolaryngol 81:743, 1976 29. Slingsby JG, Forstot SL: Effect of blunt trauma on the corneal endothelium. Arch Ophthalmol 99:1041, 1981 30. Beyer TL, Hirst LW: Corneal blood staining at low pressures. Arch Ophthalmol 103:654, 1985 31. Read J, Goldberg MF: Comparison of medical treatment for hyphema. Trans Am Acad Acad Ophthalmol Otolaryngol 78:799, 1974 32. Tonjum A: Gonioscopy in traumatic hyphema. Acta Ophthalmol 44:650, 1966 33. Tanaka S, Takeuchi S, Ideta H: Ultrasound biomicroscopy for detection of breaks and detachment of the
ciliary epithelium. Am J Ophthalmol 128:466, 1999 34. Genovesi-Ebert F, Rizzo S, Chiellini S, et al: Ultrasound biomicroscopy in the assessment of penetrating or blunt anterior-chamber
trauma. Ophthalmologica 212:6, 1998 35. Park M, Kondo T: Ultrasound biomicroscopic findings in a case of cyclodialysis. Ophthalmologica 212:194, 1998 36. Assia EI, Blotnick CA, Powers TP, et al: Clinicopathologic study of ocular trauma in eyes with intraocular lenses. Am J Ophthalmol 117:30, 1994 37. Agrawal V, Wagh M, Krishnamachary M, et al: Traumatic wound dehiscence after penetrating keratoplasty. Cornea 14:601, 1995 38. Bloom HR, Sands J, Schneider D: Corneal rupture from blunt trauma 22 months after radial keratotomy. Refract Corneal Surg 6:197, 1990 39. Walton W, Von Hagen S, Grigorian , et al: Management of traumatic hyphema. Surv Ophthalmol 47:297, 2002 40. Yanoff M, Fine B: Ocular Pathology: a Text and Atlas. Hagerstown, MD: Harper & Row, 1975:145–148 41. Sihota R, Sood NN, Agarwal HC: Traumatic glaucoma. Acta Ophthalmol Scand 73:252, 1995 42. Kaufman J, Tolpin D. Glaucoma after traumatic angle recession. Am J Ophthalmol 78:648, 1974 43. Mermoud A, Salmon JF, Barron A, et al: Surgical management of post-traumatic angle recession glaucoma. Ophthalmology 100:634, 1993 44. Zabriskie NA, Hwang IP, Ramsey JF, et al: Anterior lens capsule rupture caused by air bag trauma. Am J Ophthalmol 123:832, 1997 45. Rao SK, Parikh S, Padhmanabhan P: Isolated posterior capsule rupture in blunt trauma: pathogenesis and management. Ophth Surg Lasers 29:338, 1998 46. Ikeda N, Ikeda T, Nagata M, et al: Pathogenesis of transient high myopia after blunt eye trauma. Ophthalmology 109:501, 2002 47. Doganay S, Hepsen IF, Evereklioglu C: Bilateral myopia following blunt trauma to one eye. Eur J Ophthalmol 11:83, 2001 48. Rosenbaum JT, Tammaro J, Robertson JE: Uveitis precipitated by nonpenetrating ocular trauma. Am J Ophthalmol 112:392, 1991 49. Nasrullah A, Kerr NC: Sickle cell trait as a risk factor for secondary hemorrhage in children
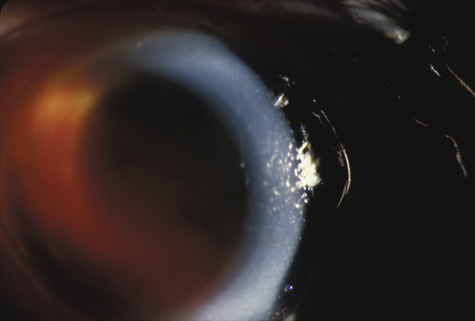
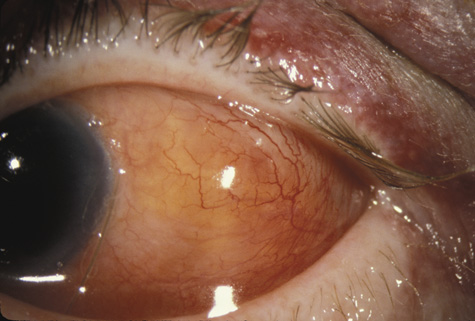
with traumatic hyphema. Am J Ophthalmol 123:783, 1997 50. Recchia F, Saluja R, Hammel K, et al: Outpatient management of traumatic microhyphema. Ophthalmology 109:1465, 2002 51. Shiuey Y, Lucarelli M: Traumatic hyphema: outcomes of outpatient management. Ophthalmology 105:851, 1998 52. Gilbert HD, Jensen AD: Atropine in the treatment of traumatic hyphema. Ann Ophthalmol, 5:1297, 1973 53. Ohrstrom A: Treatment of traumatic hyphema with corticosteroids and mydriatics. Acta Ophthalmol 50:549, 1972 54. Bedrossian RH: The management of traumatic hyphema. Ann Ophthalmol, 6:1016, 1974 55. Spoor TC, Hammer M, Belloso H: Traumatic hyphema: Failure to alter its course: a double-blind prospective
study. Arch Ophthalmol, 98:116, 1980 56. Farber MD, Fiscella R, Goldberg MF: Aminocaproic acid versus prednisone for the treatment of traumatic hyphema. A
randomized clinical trial. Ophthalmology 98:279, 1991 57. Palmer DJ, Goldberg MF, Frenkel M, et al: A comparison of two dose regimens of epsilon aminocaproic acid in the prevention
and management of secondary traumatic hyphemas. Ophthalmology 93:102, 1986 58. Crouch ERJ, Frenkel M: Aminocaproic acid in the treatment of traumatic hyphema. Am J Ophthalmol 81:355, 1976 59. McGetrick JJ, Jampol LM, Goldberg MF, et al: Aminocaproic acid decreases the risk of secondary hemorrhage after traumatic
hyphema. Arch Ophthalmol 101:1031, 1983 60. Dieste MC: Intraocular pressure incrase associated with epsilon-aminocaproic acid
therapy for traumatic hyphema. Am J Ophthalmol 106:383, 1988 61. Crouch ER, Williams P, Gray MK, et al: Topical aminocaproic acid in the treatment of traumatic hyphema. Arch Ophthalmol 115:1106, 1997 62. Pieramici DJ, Goldberg MF, Melia M, et al: A phase III, multicenter, randomized, placebo-controlled clinical trial
of topoical aminocaproic acid in the management of traumatic hyphema. Ophthalmology 110:2106, 2003 63. Karkhaneh R, Naeeni M, Chams H, et al: Topical aminocaproic acid to prevent rebleeding in cases of traumatic hyphema. Eur J Ophthalmol 13:57, 2003 64. Benner JD: Transcorneal oxygen therapy for glaucoma associated with sickle cell hyphema. Am J Ophthalmol 130:514, 2000 65. Graul TA, Ruttum MS, Lloyd MA, et al: Trabeculectomy for traumatic hyphema with increased intraocular pressure. Am J Ophthalmol 117:155, 1994 66. Meyer CH, Rodrigues EB, Mennel S: Acute commotion retinae determined by cross-sectional optical coherence
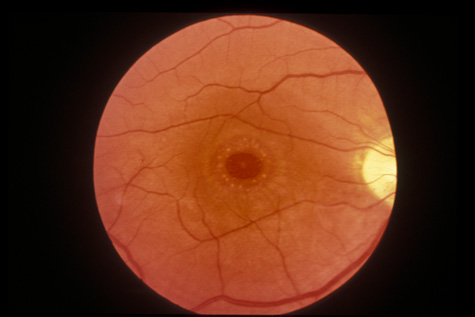
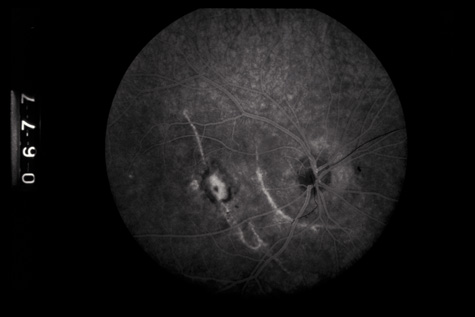
tomography. Eur J Ophthalmol 13:818, 2003 67. Blight R, Hart JCD. Structural changes in the outer retinal layers following blunt mechanical
nonperforating trauma to the globe. An experimental study. Br J Ophthalmol 61:573, 1977 68. Mansour AM, Green WR, Hogge C: Histopathology of commotio retinae. Retina 12:24, 1992 69. Sipperley JO, Quigley HA, Gass JDM: Traumatic retinopathy in primates, the explanation of commotio retinae. Arch Ophthalmol 96:2267, 1978 70. Friberg TR: Traumatic retinal pigment epithelial edema. Am J Ophthalmol 88:18, 1979 71. Bastek JV, Foos RY, Heckenlively J: Traumatic pigmentary retinopathy. Am J Ophthalmol 92:621, 1981 72. Crouch JER, Apple DER: Posttraumatic migration of retinal pigment epithelial melanin. Am J Ophthalmol 78:251, 1974 73. Al-Abdulla NA, Thompson JT, Sjaarda RN: Results of macular hole surgery with and without epiretinal dissection
or internal limiting membrane removal. Ophthalmology 111:142, 2004 74. Yamashita T, Uemara A, Uchino E, et al: Spontaneous closure of traumatic macular hole. Am J Ophthalmol 133:230, 2002 75. Johnson R, McDonald HR, Lewis H, et al: Traumatic macular hole: observations, pathogenesis, and results of vitrectomy
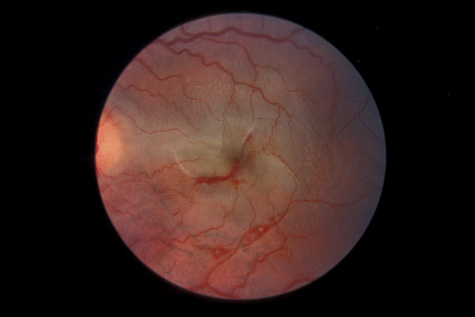
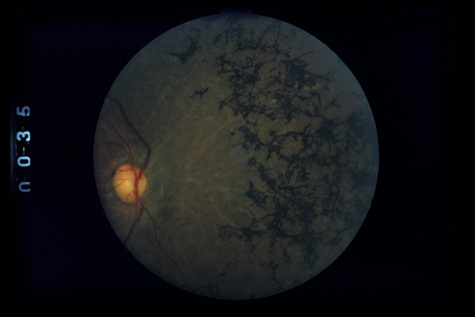
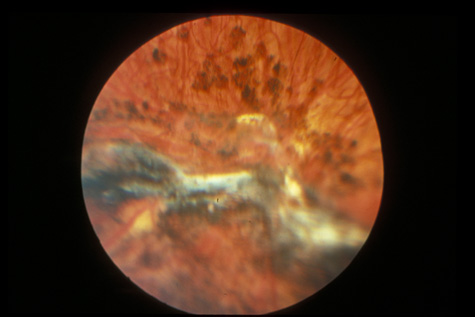
surgery. Ophthalmology 108:853, 2001 76. Weidenthal DT, Schepens CL: Peripheral fundus changes associated with ocular contusion. Am J Ophthalmol 62:465, 1966 77. Jungschaffer OH: Arrowhead tear in the macula. Arch Ophthalmol 86:19, 1972 78. Hagler WS, North AW: Retinal dialysis and retinal detachment. Arch Ophthalmol 79:376, 1968 79. Ross WH: Retinal dialysis: lack of evidence for a genetic cause. Can J Ophthalmol 26:309, 1991 80. Smiddy WE, Green WR: Retinal dialysis, pathology and pathogenesis. Retina 2:94, 1982 81. Tasman W: Peripheral retinal change following blunt trauma. Trans Am Ophthalmol Soc 70:190, 1972 82. Sellors PJ, Mooney D: Fundus changes after traumatic hyphema. Br J Ophthalmol 57:600, 1973 83. Doden W, Stark N: Retina and vitreous findings after serious indirect trauma. Klin Monatsbl Augenheilkd 164:32, 1974 84. Cox MS, Schepens CL: Retinal detachment due to ocular contusion. Arch Ophthalmol 76:678, 1966 85. Winslow RL, Tasman W: Juvenile rhegmatogenous retinal detachment. Trans Am Acad Ophthalmol Otolaryngol 85:607, 1978 86. Raman SV, Desai UR, Andrerson S, et al: Visual prognosis in patients with traumatic choroidal rupture. Can J Ophthalmol 39:260, 2004 87. Fuller B, Gitter K: Truamatic choroidal rupture with late serous detachment of the macula. Arch Ophthalmol 89:354, 1973 88. Hilton GF: Late serosanguinous detachment of the macula after traumatic choroidal
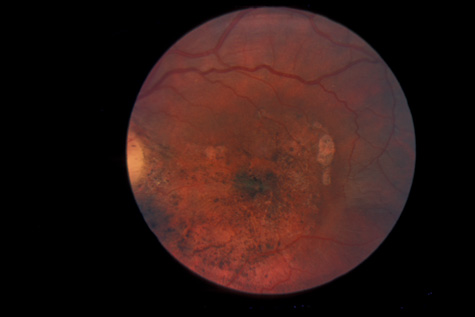
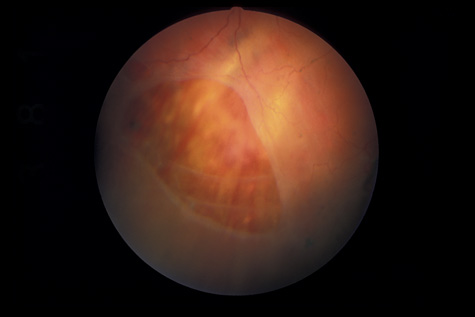
rupture. Am J Ophthalmol 79:977, 1975 89. Smith RE, Kelley JS, Harbin TS: Late macular complications of choroidal rupture. Am J Ophthalmol 77:650, 1974 90. Rogers AH, Duker JS, Nichols N: Photodynamic therapy of idiopathic and inflammatory choroidal neovascularization
in young adults. Ophthalmology 110:1315, 2003 91. Sickenberg M, Schmidt-Erfurth U, Miller J: A preliminary study of photodynamic therapy using verteporfin for choroidal
neovascularization in pathologic myopia, ocular histoplasmosis syndrome, angioid
streaks, and idiopathic causes. Arch Ophthalmol 117:327, 2000 92. Kylstra JA, Lamkin JC, Runyan DK: Clinical predictors of scleral rupture after blunt ocular trauma. Am J Ophthalmol 115:530, 1993 93. Joondeph BC, Young TL, Saran BR: Multiple scleral ruptures after blunt ocular trauma. Am J Ophthalmol 108:744, 1989 94. Levin LA, Beck RW, Joseph MP, et al: The treatement of traumatic optic neuropathy: The International Optic Nerve
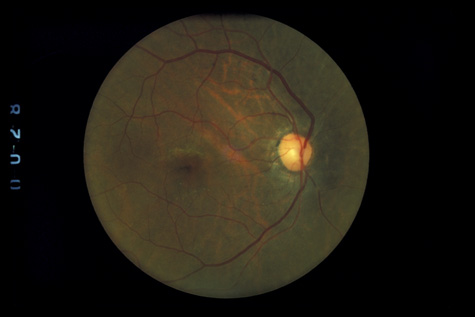
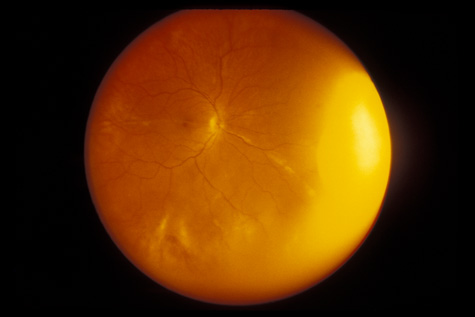
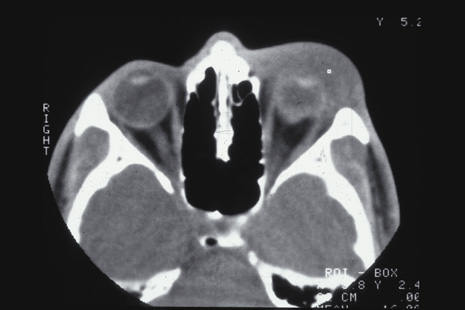
Trauma Study. Ophthalmology 106:1268, 1999 95. Steinsapir KD, Goldberg RA: Traumatic optic neuropathy. Surv Ophthalmol 38:487, 1994 96. Goldenberg-Cohen N, Miller NR, Repka MX: Traumatic optic neuropathy in children and adolescents. J AAPOS 8:20, 2004 97. Martin DF, Awh CC, McCuen BW, et al: Treatment and pathogenesis of traumatic chorioretinal rupture (sclopetaria). Am J Ophthalmol 117:190, 1994 98. Dubovy SR, Guyton DL, Green WR: Clinicopathologic correlation of chorioretinitis sclopetaria. Retina 17:510, 1997 |