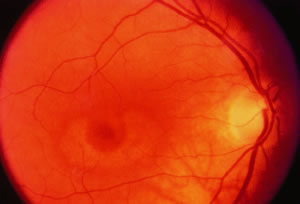
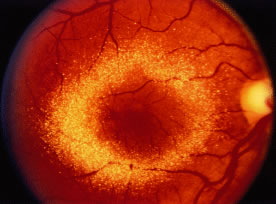
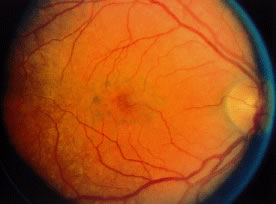
Toxic maculopathy is usually reversible only in its earliest phases. If these drugs have caused skin, eyelid, corneal (hydroxychloroquine), or hair changes, this may be an indicator of possible drug-induced retinopathy. Because the aminoquinolines are concentrated in pigmented tissue, macular changes have been thought to progress long after the drug is stopped. The bull's-eye macula is not diagnostic for aminoquinoline-induced disease because a number of other entities can cause this same clinical picture. Although retinal toxicity occurs in patients taking hydroxychloroquine, the incidence is much lower than with chloroquine.
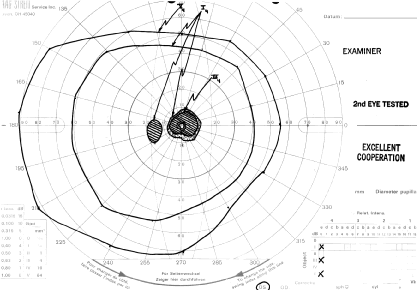
How to detect early toxic changes is still the subject of debate. Easterbrook5–8 has published extensively on chloroquine and hydroxychloroquine retinal toxicity. His current method of examination includes obtaining best corrected vision, examining the cornea with the pupil dilated with retroillumination, and performing an Amsler examination. (He has his patients test themselves every few weeks at home with an Amsler grid.) If the results of the Amsler grid examination are abnormal, a Humphrey field with the 10-2 red and white program is performed. If color vision is deficient on Ishihara testing or if there is any question of a patient's reliability in terms of visual field assessment,fluorescein angiography is done as well. According to Easterbrook, electroretinographic and electro-oculogram studies are either too variable or are abnormal only in late stages of chloroquine retinopathy, so their usefulness is suspect. Color testing is more useful in older patients in whom coincidental age-related macular changes occur. It is also stated that early retinopathy (i.e., small paracentral relative scotomas) (Fig. 2) does not appear to progress, at least in the short term. Patients who present with absolute scotomas and positive fluorescein angiography should be warned that their retinopathy may progress even if the chloroquine therapy is discontinued. There are numerous instances of progression of macular and optic nerve damage, even years after these drugs are discontinued. This may not be as true for hydroxychloroquine because the progression of the maculopathy may be significantly less than with chloroquine once the drug is stopped.
|
Some patients wish to continue taking these drugs even with the visual side effects because only these drugs improve their quality of life. If reproducible abnormalities of the Amsler grids occur in this group of patients, kinetic and static perimetry are obtained. If field defects are confirmed, consultation with the rheumatologist concerning discontinuation of the drug is advised. If the patient is reluctant, one may consider halving the usual dosages and following the patient (even those with relative paracentral scotomas with serial fields) every 3 to 4 months. If, however, there is any progression, the recommendation is to stop the drug.
OVERVIEW
Maculopathy must be bilateral and reproducible by Amsler grid and visual field testing. Transient or unilateral defects are not sufficient reasons to implicate the drug and are not an indication to stop therapy. Color vision loss is a late change. The goal is to find early changes, such as relative scotomas substantiated by visual fields. Also suspect are patients with retinal changes, color vision loss, absolute scotoma, and decreased vision, because even if the drug is stopped, two thirds of these patients may continue to lose some vision and/or peripheral fields. Patients with early paracentral relative scotomas do not advance when the drug is discontinued.
GUIDELINES FOR FOLLOWING PATIENTS
Guidelines for follow-up of patients are controversial. Morsman and colleagues,9 Morand and colleagues,2 and Levy10 question the need to screen patients on hydroxychloroquine. Spalton11 suggests every 3 years and Levy10 suggests after 10 years. Blyth and Lane12 suggest ophthalmic examination only after the patient becomes symptomatic. We favor the approach by Easterbrook that is presented here.
- Initial examination. We prefer baseline complete eye examination. This includes visual acuity, Amsler
grids, color vision, and corneal retroillumination. If abnormalities
are in the Amsler grid, automated perimetry is indicated.
- Follow-up examinations. Hydroxychloroquine—Ophthalmic examination, repeating previously
mentioned baseline studies every 12 to 18 months if the patients are taking
less than 6.5 mg/kg (ideal body weight) with normal kidney and/or
liver function. This is in keeping with the American Academy of Ophthalmology's
recommendation for biannual eye examination in normal
adults. If the patient has been taking the drug for more than 6 years
or if the accumulative dose is 200 g, patients should be examined at
least annually. Patients should see the ophthalmologist if there is any
change in vision or if changes on their “home” Amsler grid
testing are noticed. Chloroquine—The previously described tests
are performed. The patient should be seen at least annually if dose
is less than 3.0 mg/kg (ideal body weight). Patients should be seen every 6 months
if dose is greater than 3.0 mg/kg (ideal body weight) or
if they are short, are obese, or have renal and/or liver impairment.
- American Academy of Ophthalmology recommendations are pending and will
be similar to what we have described.
TESTS
- Amsler grids appear to be the most cost-effective method of following patients. Monthly
home testing is essential, especially if the patient is on higher
doses or long-term therapy or has renal disease.
- Routine visual fields are not necessary unless Amsler field defects, abnormal color vision, decreased
vision, or abnormal retinal findings are detected. A baseline
visual field has been advocated by some.
- Retroillumination of the cornea with the slit lamp with dilated pupils to look for enhanced Hudson-Stähli
line, or more commonly, whorl-like superficial corneal deposits; even
if faint, these are early indicators of possible retinal toxicity
in hydroxychloroquine patients.
CAUTION
To date, there are no data to show hydroxychloroquine toxicity worsening pre-existing macular degeneration. Common sense may make informed consent and explanation of risk-benefit ratios necessary on an individualized basis.