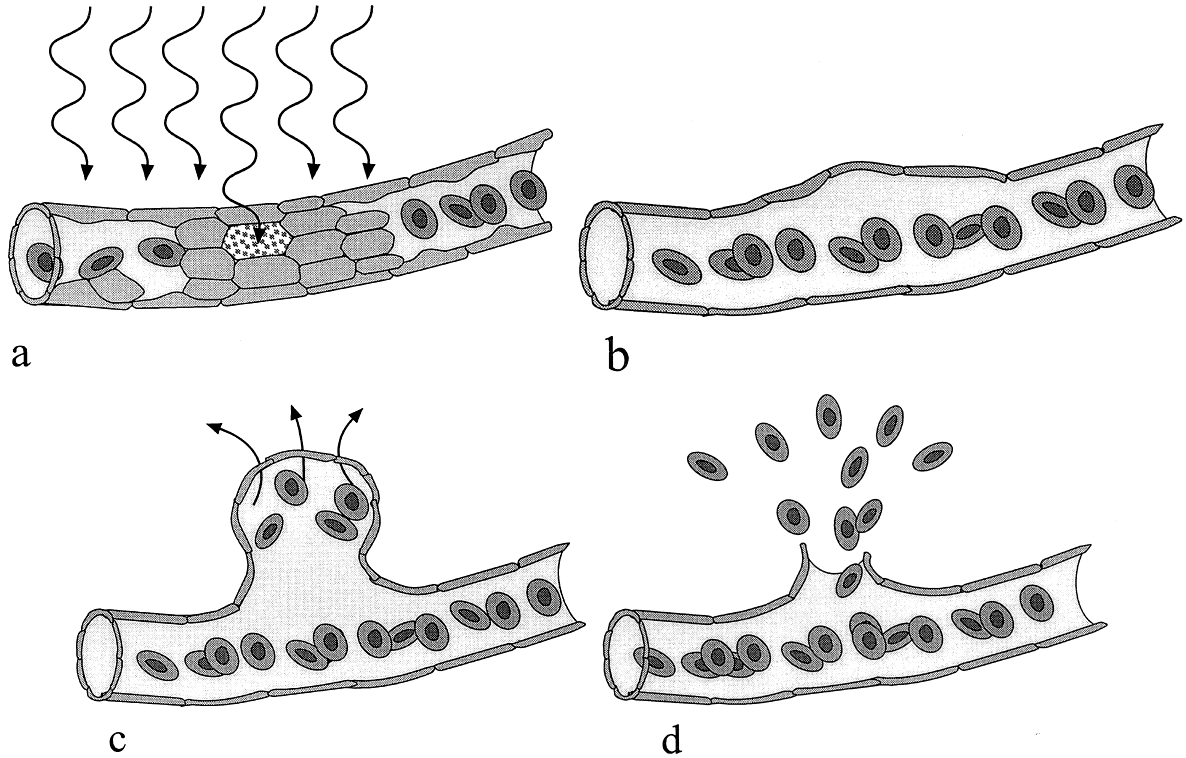
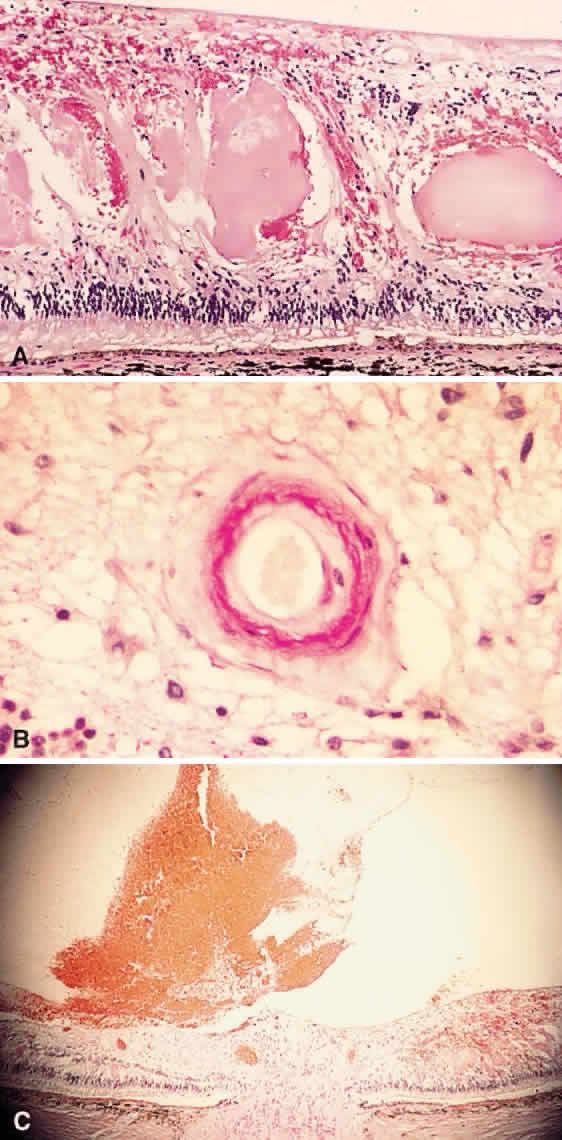
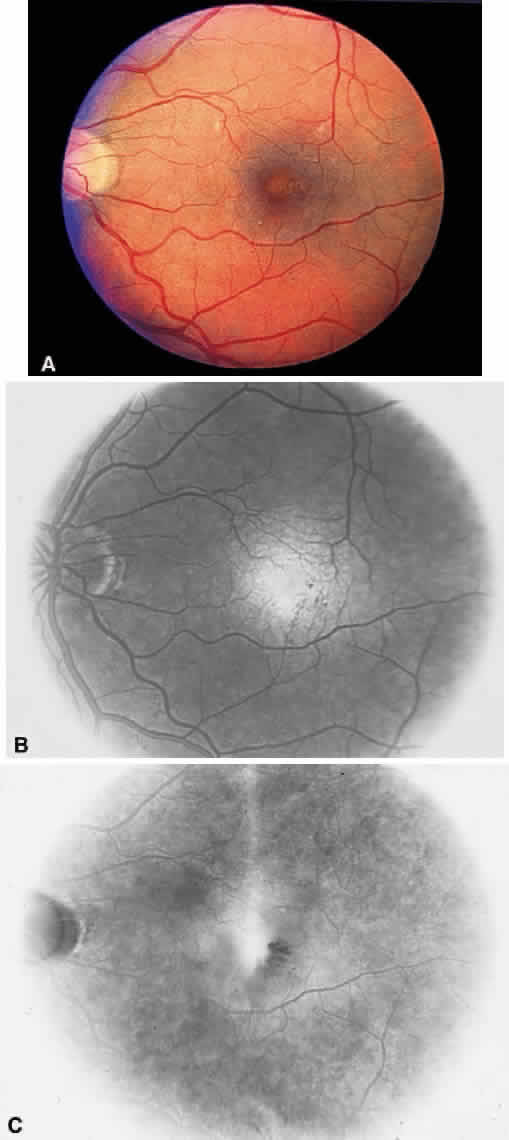
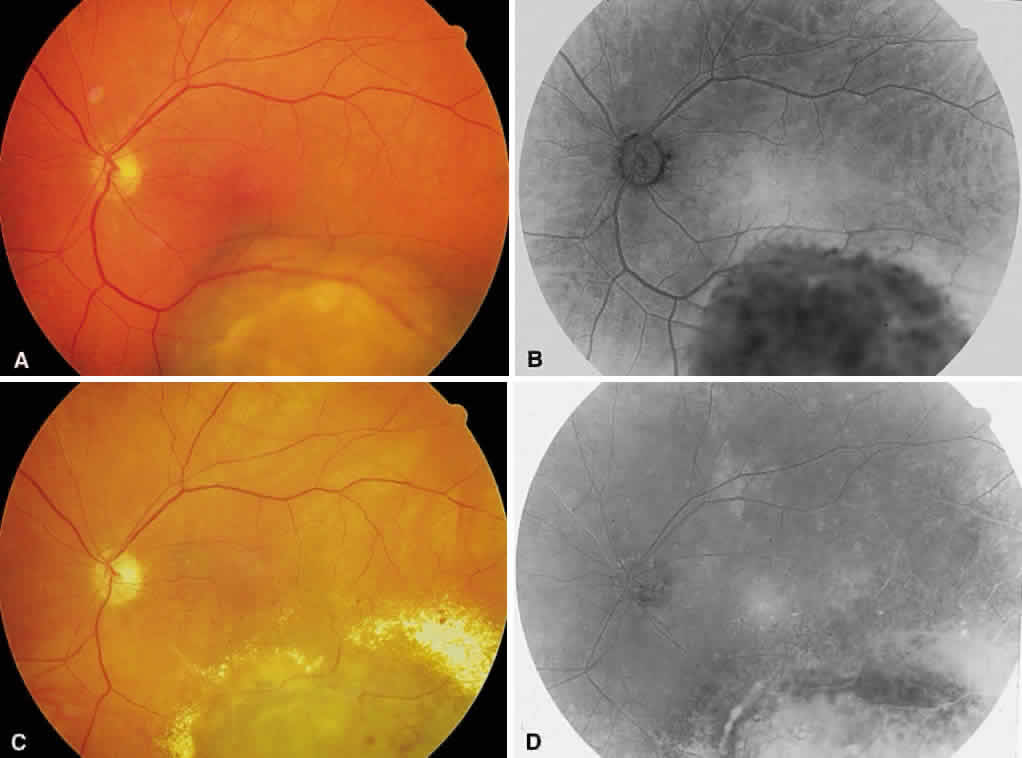
1. Stallard H: Radiant energy as (a) a pathogenic (b) a therapeutic agent in ophthalmic
disorders. Br J Ophthalmol Monogr 6(suppl):1, 1933 2. Brown GC, Shields JA, Sanborn G et al: Radiation retinopathy. Ophthalmology 89:1494, 1982 3. Archer DB, Amoaku WMK, Gardiner TA: Radiation retinopathy: Clinical, histopathological, ultrastructural, and
experimental correlations. Eye 5:239, 1991 4. Schachat AP: Radiation retinopathy. In Ryan SJ (ed): Retina, p 541. St
Louis, CV Mosby, 1989 5. Egbert PR, Farjado LF, Donaldson SS, Moazed K: Posterior ocular abnormalities after irradiation for retinoblastoma: A
histopathological study. Br J Ophthalmol 64:660, 1980 6. Brady LW, Shields JA, Augsburger JJ et al: Complications from radiation
therapy to the eye. In Vaeth JM, Meyer JL (eds): Radiation Tolerance
of Normal Tissues: Frontiers of Radiation Therapy and Oncology, p 239. Basel, Karger, 1989 7. Ross HS, Rosenberg S, Friedman AH: Delayed radiation necrosis of the optic nerve. Am J Ophthalmol 76:683, 1973 8. Nakissa N, Rubin P, Strohl R, Keys H: Ocular and orbital complications following radiation therapy of paranasal
sinus malignancies and review of literature. Cancer 51:980, 1983 9. Irvine AR, Wood IS: Radiation retinopathy as an experimental model for ischemic proliferative
retinopathy and rubeosis iridis. Am J Ophthalmol 103:790, 1987 10. Archer DB, Amoaku WMK, Gardiner TA: Radiation retinopathy: Clinical, histopathological, ultrastructural, and
experimental correlations. Eye 5:239, 1991 11. Cogan DG: Lesions of the eye from radiant energy. JAMA 142:145, 1950 12. Shukovsky LJ, Fletcher GH: Retinal and optic nerve complications in a high dose irradiation technique
of ethmoid sinus and nasal cavity. Radiology 104:629, 1972 13. Guyer DR, Mukai S, Eagan KM et al: Radiation maculopathy following proton beam irradiation for choroidal melanoma. Ophthalmology 99:1278, 1992 14. Flick JJ: Ocular lesions following the atomic bombing of Hiroshima and Nagasaki. Am J Ophthalmol 31:137, 1948 15. Chee PHY: Radiation retinopathy. Am J Ophthalmol 66:860, 1968 16. Hayreh SS: Post-radiation retinopathy: A fluorescence fundus angiographic study. Br J Ophthalmol 54:705, 1970 17. MacFaul PA, Bedford MA: Ocular complications after therapeutic irradiation. Br J Ophthalmol 54:237, 1970 18. Augsburger JJ: Radiation retinopathy. In Tasman W (ed): Clinical Decisions
in Medical Retina, p 261. St Louis, Mosby, 1994 19. Brown GC, Shields JA, Sanborn G et al: Radiation optic neuropathy. Ophthalmology 89:1489, 1982 20. Rosengren B: Two cases of atrophy of the optic nerve after previous roentgen treatment
of the chiasmal region and the optic nerves. Acta Ophthalmol 36:874, 1958 21. Gass JDM: A fluorescein angiographic study of macular dysfunction secondary to retinal
vascular disease. VI. X-ray irradiation, carotid artery occlusion, collagen
vascular disease, and vitritis. Arch Ophthalmol 80:606, 1968 22. Amoaku WMK, Archer DB: Fluorescein angiographic features, natural course and treatment of radiation
retinopathy. Eye 4:657, 1990 23. Chaudhuri PR, Austin DJ, Rosenthal AR: Treatment of radiation retinopathy. Br J Ophthalmol 65:623, 1981 24. Kinyoun JL, Chittum ME, Wells CG: Photocoagulation treatment of radiation retinopathy. Am J Ophthalmol 105: 470, 1988 25. Midena E, Segato T, Valenti M et al: The effect of external eye irradiation on choroidal circulation. Ophthalmology 103: 1651, 1996 26. Irvine AR, Alvarado JA, Wara WM et al: Radiation retinopathy: An experimental model for the ischemicóproliferative
retinopathies. Trans Am Ophthalmol Soc 79:103, 1981 27. Takahashi K, Kishi S, Muraoka K et al: Radiation choroidopathy with remodeling of the choroidal venous system. Am J Ophthalmol 125:367, 1998 28. Kinyoun JL, Kalina RE, Brower SA et al: Radiation retinopathy after orbital irradiation for Gravesí ophthalmopathy. Arch Ophthalmol 102:1473, 1984 29. Bagan SM, Hollehorst RW: Radiation retinopathy after irradiation of intracranial lesions. Am J Ophthalmol 88:694, 1979 30. Midena E, Segato T, Piermarocchi S et al: Retinopathy following radiation therapy of paranasal sinus and nasopharyngeal
carcinoma. Retina 7:142, 1987 31. Parsons T, Bova FJ, Fitzgerald CR et al: Radiation retinopathy after external-beam irradiation: Analysis of time-dose
factors. Int J Radiat Oncol Biol Phys 30:765, 1994 32. Mewis L, Tang RA, Salmonsen PC: Radiation retinopathy after “safe” levels of irradiation. Invest Ophthalmol Vis Sci 22:222, 1982 33. Lopez PF, Sternberg P, Dabbs CK: Bone marrow transplant retinopathy. Am J Ophthalmol 112:635, 1991 34. Viebahn M, Barricks ME, Osterloh MD: Synergism between diabetic and radiation retinopathy: Case report and review. Br J Ophthalmol 75:629, 1991 35. Kinyoun JL, Zamber RW, Lawrence BS et al: Photocoagulation treatment for clinically significant radiation macular
oedema. Br J Ophthalmol 79:144, 1995 36. ETDRS Research Group: Photocoagualation of diabetic macular edema. Arch
Ophthalmol 103:1796, 1985 37. ETDRS Research Group: Treatment techniques and clinical guidelines for
photocoagulation of diabetic macular edema. Ophthalmology 94:761, 1987 38. Kinyoun JL, Lawrence SB, Barlow WE: Proliferative radiation retinopathy. Arch Ophthalmol 114:1097, 1996 39. Diabetic Retinopathy Study Research Group: Photocoagulation treatment of
proliferative diabetic retinopathy. Ophthalmology 85:82, 1978 40. Guy J, Schatz NJ: Hyperbaric oxygen in the treatment of radiation-induced optic neuropathy. Ophthalmology 93: 1083, 1986 41. Roden D, Bosley Tm, Fowble B: Delayed radiation injury to the retrobulbar optic nerves and chiasm. Ophthalmology 97:346, 1990 42. Harris JR, Levene MB: Visual complications following irradiation for pituitary adenomas and craniopharyngiomas. Radiology 120:167, 1976 43. Aristizabal S, Caldwell WL, Avila J: The relationship of time-dose fractionation factors to complications in
the treatment of pituitary tumors by irradiation. Int J Radiat Oncol Biol Phys 2:667, 1977 |