1. Plato: Dialogues of Plato. In Encyclopedia Britannica Great Book Series. Chicago, Phaedo
Press, 1952 2. Sliney DH, Wolbarsht ML: Safety With Lasers and Other Optical Sources. New
York, Plenum Publishing, 1980 3. Mainster MA, Ham WT Jr, Delori FC: Potential retinal hazards: Instrument and environmental light sources. Ophthalmology 90:927, 1983 4. Thach AB, Lopez PF, Snady-McCoy LC et al: Accidental Nd:YAG laser injuries to the macula. Am J Ophthalmol 119:767, 1995 5. Ham WT Jr, Ruffolo JJ Jr, Mueller HA et al: The nature of retinal radiation damage: Dependence on wavelength, power
level and exposure time. Vis Res 20:1105, 1980 6. Mainster MA, White TJ, Tips JH, Wilson PW: Retinal-temperature increases produced by intense light sources. J Opt Soc Am 60:264, 1970 7. Mainster MA: Wavelength selection in macular photocoagulation: Tissue optics, thermal
effects, and laser systems. Ophthalmology 93:952, 1986 8. Michels M, Sternberg P Jr: Operating microscope-induced retinal phototoxicity: Pathophysiology, clinical
manifestations and prevention. Surv Ophthalmol 34:237, 1990 9. McDonald HR, Irvine AR: Light-induced maculopathy from the operating microscope in extracapsular
cataract extraction and intraocular lens implantation. Ophthalmology 90:945, 1983 10. Ham WT Jr, Mueller HA, Ruffolo JJ Jr et al: Sensitivity of the retina to radiation damages as a function of wavelength. Photochem Photobiol 29:735, 1979 11. Mainster MA: Finding your way in the photoforest: Laser effects for clinicians. Ophthalmology 91:886, 1984 12. Ham WT Jr, Mueller HA, Sliney DH: Retinal sensitivity to damage from short wavelength light. Nature 260:153, 1976 13. Ham WT Jr, Mueller HA, Ruffolo JJ Jr et al: Basic mechanisms underlying the production of photochemical lesions in
the mammalian retina. Curr Eye Res 3:165, 1984 14. Lawhill T: Three major pathologic processes caused by light in the primate retina: A
search for mechanisms. Trans Am Ophthalmol Soc 80:517, 1982 15. Noell WK: Possible mechanisms of photoreceptor damage by light in mammalian eyes. Vision Res 20:1163, 1980 16. Tso MOM: Photic maculopathy in rhesus monkeys: A light and electron microscopic
study. Invest Ophthalmol Vis Sci 12:17, 1973 17. Birch-Hirschfeld A: Zum Kapitel der sonnenblendung des auges. Z Augenheilkd 28:324, 1912 18. Verhoeff FH, Bell L: The pathological effects of radiant energy on the eye. Proc Am Acad Arts Sci 51:630, 1916 19. Vos JJ: A theory of retinal burns. Bull Math Biophys 24:115, 1962 20. Mainster MA, White TJ, Allen RG: Spectra dependence of retinal damage produced by intense light sources. J Opt Soc Am 50:848, 1970 21. Mainster MA, White TJ, Tips JH et al: Retinal-temperature increases produced by intense light sources. J Opt Soc Am 60:262, 1970 22. White TJ, Mainster MA, Wilson PW et al: Chorioretinal temperature increases from solar observation. Bull Math Biophys 33:1, 1971 23. Cain CP, Welch AJ: Measured and predicted laser induced temperature rise in the rabbit fundus. Invest Ophthalmol 13:60, 1974 24. Priebe LA, Cain CP, Welch AJ: Temperature rise required for the production of minimal lesions in the
macaca mulatta retina. Am J Ophthalmol 79:405, 1975 25. Noell WK, Walker VS, Kang BS et al: Retinal damage by light in rats. Invest Ophthalmol 5:450, 1966 26. Lanum J: The damaging effects of light on the retina: Empirical findings, theoretical
and practical implications. Surv Ophthalmol 22:221, 1978 27. Sperling HG: Prolonged intense spectral light effects on rhesus retina. In
Williams TP, Baker BN (eds): The Effects of Constant Light on Visual
Processes, pp 195–214. New York, Plenum, 1979 28. Ham WT Jr, Ruffolo JJ Jr, Mueller HA et al: Histologic analysis of photochemical lesions produced in rhesus retina
by short wavelength light. Invest Ophthalmol Vis Sci 17:1029, 1978 29. Lawhill T, Crockett S, Currier G: Retinal damage secondary to chronic light exposure, thresholds and mechanisms. Doc Ophthalmol 44:379, 1977 30. Hochheimer BF, d'Anna SA, Calkins JL: Retinal damage from light. Am J Ophthalmol 88:1039, 1979 31. Robertson DM, Feldman RB: Photic retinopathy from the operating room microscope. Am J Ophtlalmol 101:561, 1986 32. DeLaey JJ, DeWachter A, VanOye R et al: Retinal phototrauma during intraocular lens implantation. Int Ophthalmol 7:109, 1984 33. Khwarg SG, Geoghegan M, Hanscom TA: Light induced maculopathy from the operating microscope. Am J Ophthalmol 98:628, 1984 34. Khwarg SG, Linstone FA, Daniels SA et al: Incidence, risk factors and morphology in operating microscope light retinopathy. Am J Ophthalmol 103:255, 1987 35. Berler DK, Peyser R: Light intensity and visual acuity following cataract surgery. Ophthalmology 90:933, 1983 36. Boldrey EE, Ho BT, Griffith RD: Retinal burns occurring at cataract extraction. Ophthalmology 91:1297, 1984 37. Lindquist TD, Grutzmacher RD, Gofman JD: Light-induced maculopathy: Potential for recovery. Arch Ophthalmol 104:1641, 1986 38. Byrnes GA, Antoszyk AN, Mazur DO et al: Photic maculopathy after extracapsular cataract surgery: A prospective
study. Ophthalmology 99:731, 1992 39. Stamler JF, Blodi CF, Verdier D et al: Microscope light-induced maculopathy in combined penetrating keratoplasty, extracapsular
cataract extraction, and intraocular lens implantation. Ophthalmology 95:1142, 1988 40. Cech JM, Choromokose EA, Sanitato JA: Light induced maculopathy following penetrating keratoplasty and intraocular
lens implantation. Arch Ophthalmol 105:751, 1987 41. McDonald HR, Harris MJ: Operating microscope induced retinal phototoxicity during pars plana vitrectomy. Arch Ophthalmol 106:521, 1988 42. Michels M, Lewis H, Abrams GW et al: Macular phototoxicity caused by fiberoptic endoillumination during pars
plana vitrectomy. Am J Ophthalmol 114:287, 1992 43. Kuhn F, Morris R, Massey M: Photic retinal injury from endoillumination during vitrectomy. Am J Ophthalmol 111:42, 1991 44. Azzolini C, Brancato R, Venturi G et al: Updating on intraoperative light-induced retinal injury. Int Ophthalmol 18:269, 1995 45. Sliney DH: Eye protective techniques for bright light. Ophthalmology 90:937, 1983 46. Snodderly DM, Auran JD, Delori FC: The macular pigment. II. Spatial distribution in primate retinas.Invest Ophthalmol Vis Sci 25:674, 1984 47. Kirschfeld K: Carotenoid pigments. Their possible role in protecting against photooxidation
in eyes and photoreceptor cells.Proc R Soc Lond Biol 216:71, 1982 48. Jaffe GJ, Wood I: Retinal phototoxicity from the operating microscope: Protective effect
by the fovea. Arch Ophthalmol 106:445, 1988 49. Rapp LM, Williams TP: The role of ocular pigmentation in protecting against retinal light damage. Vision Res 20:127, 1980 50. Young RW: The Bowman Lecture: Biological renewal: Applications to the eye. Trans Ophthalmol Soc UK 102:42, 1982 51. Fridovich I: Oxygen radicals, hydrogen peroxide, and oxygen toxicity. In
Pryor WA (ed): Free Radicals in Biology, pp 239–277. New York, Academic
Press, 1976 52. Mayer EF: Transmission and absorption coefficients for ocular media of
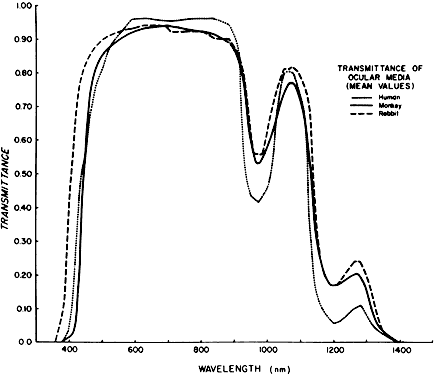
the rhesus monkey, report SAM-TR-78-32. Brook Air Force Base, TX, USAF
School of Aerospace Medicine, 1978 53. Weast RC (ed): CRC Handbook of Chemistry and Physics: A Ready Reference
Book of Clinical and Physical Data, p 184. Boca Raton, FL, CRC Press, 1984 54. Boettner EA, Wolter JR: Transmission of ocular media. Invest Ophthalmol Vis Sci 1:776, 1962 55. Lerman S: Chemical and physical properties of the normal and aging lens: Spectroscopic (UV, fluorescence, phosphorescence and NMR) analyses. Am J Optom Physiol Optics 64:11, 1987 56. Brancato R, Pratesi R: Application of diode laser in ophthalmology. Laser Ophthalmol 1:119, 1987 57. Azzolini C, Docchio F, Brancato R et al: Interactions between light and vitreous fluid substitutes. Arch Ophthalmol 110:1468, 1992 58. Ham WT, Mueller HA, Ruffolo JJ et al: Solar retinopathy as a function of
wavelength: Its significance for protective eyewear. In Williams TP, Baker
BN (eds): The Effects of Constant Light on Visual Processes, pp 319–346. New
York, Plenum Press, 1980 59. Geeraets WJ, Berry ER: Ocular spectral characteristics as related to hazards from laser and other
light sources. Am J Ophthalmol 66:15, 1968 60. Ham WT, Ruffolo JJ, Mueller HA et al: Action spectrum of retinal injury from near UV radiation in the aphakic
monkey. Am J Ophthalmol 93:299, 1982 61. Lawhill T: Effects of prolonged exposure of rabbit retina to low intensity light. Invest Ophthalmol Vis Sci 12:45, 1973 62. Flynn HW, Brod RD: Protection from operating microscope-induced retinal phototoxicity during
pars plana vitrectomy. Arch Ophthalmol 106:1032, 1988 63. Brod RD, Olson KR, Ball SF et al: The site of operating microscope light induced injury on the human retina. Am J Ophthalmol 207:390, 1989 64. Friedman E, Kuwabara T: Retinal pigment epithelium: Damaging effects of radiant energy. Arch Ophthalmol 80:265, 1968 65. Rinkoff J, Machemer R, Hida T et al: Temperature dependent light damage to the retina. Am J Ophthalmol 102:452, 1986 66. Parver LM, Ham WT, Mitchard et al: Arterial oxygen levels as a risk factor for photochemical retinal damage
in the surgical eye patient. Ophthalmology 91(suppl):135, 1984 67. Irvine AR: Retinal complications of cataract surgery. In Freeman WR (ed): Practical
Atlas of Retinal Disease and Therapy, pp 155–161. Philadelphia, Lippincott-Raven, 1998 68. Irvine AR, Wook I, Morris AW: Retinal damage from the illumination of the operating microscope: An experiment
and study in pseudophakic monkeys. Arch Ophthalmol 102:1358, 1984 69. Colvard DM: Operating microscope light-induced retinal injury: Mechanisms, clinical
manifestation and preventive measures. Am Intraocular Implant Soc J 10:438, 1984 70. Griess GA, Blankenstein MF: Additivity and repair of actinic retinal lesions. Invest Ophthalmol Vis Sci 20:803, 1981 71. Roberts JE, Reme CE, Dillon J et al: Exposure to bright light and the concurrent use of photosensitizing drugs. N Engl J Med 326:1500, 1992 72. Davidson PC, Sternberg P Jr: Potential retinal phototoxicity. Am J Ophthalmol 116:497, 1993 73. Cowan CL: Light hazards in the operating room. J Natl Med Assoc 84:425, 1992 74. Borsje RA, Vrensen GF, van Best JA et al: Fluorophotometric assessment of blood-retinal barrier function after white
light exposure in the rabbit eye. Exp Eye Res 50:297, 1990 75. Tso MOM, Fine B, Zimmerman L: Photic maculopathy produced by the indirect ophthalmoscope. Am J Ophthalmol 73:686, 1972 76. Tso MOM, Woodward BJ: Effect of photic injury on the retinal tissues. Ophthalmology 90:952, 1983 77. Yannuzzi LA, Fisher YL, Krueger A et al: Solar retinopathy: A photobiological and geophysical analysis. Trans Am Ophthalmol Soc 85:120, 1987 78. Penner R, McNair JN: Eclipse blindness. Am J Ophthalmol 61:1452, 1966 79. Hatfield EM: Eye injuries and the solar eclipse. Sight Saving Rev 40:79, 1970 80. Cordes FC: A type of foveomacular retinitis observed in the US Navy. Am J Ophthalmol 27:803, 1944 81. Ewald RA, Ritchey CL: Sun gazing as the cause of foveomacular retinitis. Am J Ophthalmol 70:491, 1970 82. Kerr LM, Little HL: Foveomacular retinitis. Arch Ophthalmol 76:498, 1966 83. Marlor RL, Blais BR, Preston FR et al: Foveomacular retinitis, an important problem in military medicine: Epidemiology. Invest Ophthalmol Vis Sci 12:5, 1973 84. Wergeland FL Jr, Brenner EH: Solar retinopathy and foveomacular retinitis. Ann Ophthalmol 7:495, 1975 85. Naidoff MA, Sliney DH: Retinal injury from a welding arc. Am J Ophthalmol 77:663, 1974 86. Romanchuk KG, Pollak V, Schneider RJ: Retinal burn from a welding arc. Can J Ophthalmol 13:120, 1978 87. Uniat L, Olk RJ, Hanish SJ: Welding arc maculopathy. Am J Ophthalmol 102:394, 1986 88. Wurdemann HV: The formation of a hole in the macula: Light burn from exposure to electric
welding. Am J Ophthalmol 19:457, 1936 89. Henry MM, Henry LM, Henry LM: A possible cause of chronic cystic maculopathy. Ann Ophthalmol 9:455, 1977 90. Macy JI, Baerveldt G: Pseudophakic serous maculopathy. Arch Ophthalmol 101:228, 1983 91. Fishman GA: Light-induced maculopathy from surgical microscopes during
cataract surgery. In Ernest JT (ed): 1985 Year Book of Ophthalmology, pp 177–181. Chicago, Year Book Medical Publishers, 1985 92. Byrnes GA, Chang B, Loose I et al: Prospective incidence of photic maculopathy after cataract surgery. Am J Ophthalmol 119:231, 1995 93. Gomolin JES, Koenekoop RK: Presumed photic retinopathy after cataract surgery: An angiographic study. Can J Ophthalmol 28:221, 1993 94. Brod RD, Barron BA, Suelflow JA et al: Phototoxic retinal damage during refractive surgery. Am J Ophthalmol 102:121, 1986 95. Kramer T, Brown R, Lynch M et al: Molteno implants and operating microscope-induced retinal phototoxicity: A
clinicopathologic report. Arch Ophthalmol 109:379, 1991 96. Rouland JF, Constantinides G, Turut P: Light-induced maculopathy following epikeratoplasty. Refractive Corneal Surg 6:270, 1990 97. Kelly NE, Wendell RT: Vitreous surgery for idiopathic macular holes: Results of a pilot study. Arch Ophthalmol 109:654, 1991 98. Brod RD, Ball SF, Packer AJ: A model for predicting the site of paraxial lesions secondary to “coaxial” operating
microscope illumination system. Am J Ophthalmol 104:516, 1987 99. Postel EA, Pulido JS, Byrnes GA et al: Long-term follow-up of iatrogenic phototoxicity. Arch Ophthalmol 116:753, 1998 100. Hupp SL: Delayed, incomplete recovery of macular function after photic retinal damage
associated with extracapsular cataract extraction and posterior
lens insertion. Arch Ophthalmol 105:1022, 1987 101. Leonardy NJ, Dabbs CK, Sternberg P Jr: Subretinal neovascularization after operating microscope burn. Am J Ophthalmol 109:224, 1990 102. van der Hoeve J: Eye lesions produced by light rich in ultraviolet rays: Senile cataract, senile
degeneration of the macula. Am J Ophthalmol 3:178, 1920 103. Gjessing HGA: Gibt es einen antagonismus zwischen cataracta senilis und haabscher seniler
makulaveranderungen? Z Augenheilkd 56:79, 1925 104. Gjessing HGA: Gibt es einen antagonismus zwischen cataracta senilis und haabscher seniler
makulaveranderungen? Acta Ophthalmol 31:401, 1953 105. Sperduto RD, Hiller R, Seigel D: Lens opacities and senile maculopathy. Arch Ophthalmol 99:1004, 1981 106. Chumbley LC: Impressions of eye diseases among Rhodesian blacks in Marshonland. S Afr Med J 52:316, 1977 107. Gregor Z, Joffe L: Senile macular changes in the black African. Br J Ophthalmol 62:554, 1978 108. Maltzman BA, Mulvihill MN, Greenbaum A: Senile macular degeneration and risk factors: A case control study. Ann Ophthalmol 11:1197, 1979 109. Hyman LG, Lilienfeld AM, Ferris FL III et al: Senile macular degeneration: A case control study. Am J Epidemiol 118:213, 1983 110. Weiter JJ, Delori FC, Wing GL et al: Relationship of senile macular degeneration to ocular pigmentation. Am J Ophthalmol 99:185, 1985 111. Holz FG, Piquet B, Minassian DC et al: Decreasing stromal iris pigmentation as a risk factor for age-related macular
degeneration. Am J Ophthalmol 117:19, 1994 112. Sandberg MA, Gaudio AR, Miller S et al: Iris pigmentation and extent of disease in patients with neovascular age-related
macular degeneration. Invest Ophthalmol Vis Sci 35:2734, 1994 113. La Vail MM: Eye pigmentation and constant light damage in the rat retina. In
Williams TP, Baker BN (eds): The Effects of Constant Light on Visual
Processes, pp 357–387. New York, Plenum, 1980 114. Penn JS, Howard AG, Williams TP: Light damage as a function of “light
history” in the albino rat. In La Vail MM, Hollyfield JG, Anderson
RE (eds): Retinal Degeneration: Experimental and Clinical Studies, pp 439–447. New
York, Alan R Liss, 1985 115. Rapp LM, Williams TP: A parametric study of retinal light damage in albino
and pigmented rats. In Williams TP, Baker BN (eds): The Effects of
Constant Light on Visual Processes, pp 135–139. New York, Plenum
Publishing, 1980 116. Taylor HR, Munoz B, West S et al: Visible light and risk of age-related macular degeneration. Trans Am Ophthalmol Soc 88:163, 1990 117. Taylor HR, Munoz B, West S et al: The long-term effects of visible light on the eye. Arch Ophthalmol 110:99, 1992 118. Cruickshanks KJ, Lein R, Klein BEK: Sunlight and age-related macular degeneration: The Beaver Dam Eye Study. Arch Ophthalmol 111:514, 1993 119. Gottsch JD, Bynoe LA, Harlan JB et al: Light-induced deposits in Bruch's membrane of protoporphyric mice. Arch Ophthalmol 111:126, 1993 120. Gottsch JD, Pou S, Bynoe LA et al: Hematogenous photosensitization: A mechanism for the development of age-related
macular degeneration. Invest Ophthalmol Vis Sci 31:1674, 1990 121. Gallard ER, Atherton SJ, Eldred G et al: Photophysical studies on human retinal lipofuscin. Photochem Photobiol 61:448, 1995 122. Harlan JB, Weidenthal DT, Green WR: Histologic study of a shielded macula. Retina 17:232, 1997 123. Drucker BL, Shapiro LA: Protective effect of occlusion on disciform degeneration. Ann Ophthalmol 20:118, 1988 124. Patz A, Hoeck LE, DeLaCruz E: Studies on the effect of high oxygen administration in retrolental fibroplasia: Nursury
observations. Am J Ophthalmol 35:1248, 1952 125. Glass P: Light and the developing retina. Doc Ophthalmol 74:195, 1990 126. Glass P, Avery GB, Subramanian KN et al: Effect of bright light in the hospital nursery on the incidence of retinopathy
of prematurity. N Engl J Med 313:401, 1985 127. Sadda SR, Yu YS, de Juan E Jr et al: Photosensitization-induced retinopathy in the newborn beagle. Invest Ophthalmol Vis Sci 35:1202, 1994 128. Katz ML, Robinson WG Jr: Autoxidative damage to the retina: Potential role
in retinopathy of prematurity. In Flynn JT, Phelps DL (eds): Retinopathy
of Prematurity: Problem and Challenge,pp 237–248. New York, Alan
R Liss, 1988 129. Riley PA, Slater TF: Pathogenesis of retrolental fibroplasia. Lancet ii:265, 1969 130. Nielson JC, Naash MI, Anderson RE: The regional distribution of vitamins E and C in mature and premature human
retinas. Invest Ophthalmol Vis Sci 29:22, 1988 131. Penn JS, Thum LA, Naash MI: Oxygen-induced retinopathy in the rat: Vitamins C and E as potential therapies. Invest Ophthalmol Vis Sci 33:1636, 1992 132. Bynoe LA, Gottsch JD, Sadda SR et al: An elevated hematogenous photosensitizer in the preterm infant. Invest Ophthalmol Vis Sci 34:2878, 1993 133. Robinson J, Mosely MJ, Thompson JR et al: Eyelid opening in preterm neonates. Arch Dis Child 64:943, 1989 134. Robinson J, Fielder AR: Pupillary diameter and reaction to light in preterm neonates. Arch Dis Child 65:35, 1990 135. Isenberg SJ, Molarte A, Vazquez M: The fixed and dilated pupils of premature neonates. Am J Ophthalmol 110:168, 1990 136. Robinson J, Fielder AR: Light and the immature visual system. Eye 6:166, 1992 137. Light Reduction in Retinopathy of Prematurity (LIGHT-ROP) Cooperative Group: Lack
of efficacy of light reduction in preventing retinopathy of
prematurity. N Engl J Med 338:1620, 1998 138. Calkins JL, Hochheimer BF: Retinal light exposure from operating microscopes. Arch Ophthalmol 97:2363, 1979 139. Jampol LM: Aphakic cystoid macular edema: A hypothesis. Arch Ophthalmol 103:1134, 1985 140. Jampol LM, Kraff MC, Sanders DR et al: Near UV radiation from the operating microscope and pseudophakic cystoid
macular edema. Arch Ophthalmol 103:28, 1985 141. Iliff WJ: Aphakic cystoid macular edema and the operating microscope: Is there a
connection? Trans Am Ophthalmol Soc 83:476, 1985 142. Kraff MC, Sanders DR, Jampol LM et al: Effect of an ultraviolet filtering intraocular lens on cystoid macular
edema. Ophthalmology 92:366, 1985 143. Absolon MJ: The effects of ultraviolet light on the eye. Trans Ophthalmol Soc UK 104:522, 1985 144. Green WR, Robertson DM: Pathologic findings of photic retinopathy in the human eye. Am J Ophthalomol 112:520, 1991 145. Parver LM, Auker CR, Fine BS: Observations on monkey eyes exposed to light from an operating microscope. Ophthalmology 90:964, 1983 146. Jaffe GJ, Irvine AR, Wood IS et al: Retinal phototoxicity from the operating microscope: The role of inspired
oxygen. Ophthalmology 95:1130, 1988 147. Gorn RA, Kuwabara T: Retinal damage by visible light. Arch Ophthalmol 77:115, 1967 148. Kuwabara T, Gorn RA: Retinal damage by visible light: An electron microscopic study. Arch Ophthalmol 79:69, 1968 149. Grignolo A, Orzalesi AG, Castellazzo R et al: Retinal damage by visible light in albino rats. Ophthalmologica 157:43, 1969 150. Kuwabara T: Retinal recovery from exposure to light. Am J Ophthalmol 70:187, 1970 151. Tso MOM, LaPiana FG: The human fovea after sun-gazing. Trans Am Acad Ophthalmol
Otolaryngol 79:OP 788, 1975 152. Mainster MA: Spectral transmittance of intraocular lenses and retinal damage from intense
light sources. Am J Ophthalmol 85:167, 1978 153. Lerman S: Radiant Energy and the Eye. New York, Macmillan, 1980 154. Lerman S: Parker Heath Memorial Lecture: Photosensitizing drugs and their possible
role in enhancing ocular toxicity. Ophthalmology 93:304, 1986 155. Clarke CA, Sneddon IB: Nutritional neuropathy in prisoners-of-war and internees
from Hong-Kong. Lancet i:734, 1946 156. American National Standard: Requirements for nonprescription sunglasses
and fashion eyewear. New York, American National Standards Institute, 1986 157. Borgwardt B, Fishman GA, VanderMeulen D: Spectral transmission characteristics of tinted lenses. Arch Ophthalmol 99:293, 1981 158. Fishman GA: Ocular phototoxicity: Guidelines for selecting sunglasses. Surv Ophthalmol 31:119, 1986 159. Schwartz LK, Norris JL: A corneal shield to prevent light-induced maculopathy during cataract surgery. Am J Ophthalmol 97:658, 1984 160. McIntyre DJ: Phototoxicity: The eclipse filter. Ophthalmology 92:364, 1985 161. Nevyas HJ, Nevyas JY: Surgical corneal light occluder made of black hema. Ophthalmic Surg 16:696, 1985 162. Yanoff M, Kurata F, Lamensdorf M: Inexpensive device to reduce surgical light exposure. Ophthalmology 90(suppl):137, 1983 163. Urinowski E, Cahane M, Ashkenazi I et al: Proximity-sensor dimmer device as an aid in the reduction of operating
microscope-induced retinal phototoxicity. Ophthalmic Surg 25:122, 1994 164. Fechner PU, Barth R: Effect on the retina of an air cushion in the anterior chamber and coaxial
illumination. Am J Ophthalmol 96:600, 1983 165. Zak R, Jabbour N, Brown S: The effects of retinal hypothermia on argon
blue-green laser thresholds in vitrectomized rabbit eyes. Invest Ophthalmol
Vis Sci (suppl):292, 1982 166. Ham WT Jr, Mueller HA, Guerry RK: Light damage. In Sheffield JB, Hilfer
SR (eds): Cell and Developmental Biology of the Eye: The Microenvironment
and Vision, pp 141–158. New York, Springer-Verlag, 1987 |