HISTORY AND NOMENCLATURE
In 1917, Lindberg described grayish or bluish flakes of material on the pupillary border in some patients with glaucoma.1 Vogt later hypothesized that this material represented degenerative changes of the lens capsule followed by secondary desquamation and proposed the term senile exfoliation of the lens capsule.2 Busacca argued that the exfoliative material represented deposition of material formed elsewhere in the eye rather than degenerative changes of the lens capsule.3 Dvorak-Theobald subsequently showed that exfoliative material differed histochemically from lens capsule and, to differentiate this condition from true exfoliation of the lens capsule secondary to infrared exposure, suggested the term pseudoexfoliation of the lens capsule.4 Subsequent electron microscopic studies by Ashton and associates and Bertelsen and coworkers indicate that the anterior lens capsule was directly affected in this disorder.5,6 Bertelsen and associates suggest that preequatorial lens epithelial cells produced the abnormal fibrillar substance and recommend the term fibrillopathia epitheliocapsularis.6 Eagle and colleagues, who believe that the material represented abnormal basement membrane secretions, have called this condition basement membrane exfoliation syndrome.7
The terms exfoliation syndrome and pseudoexfoliation syndrome are now most commonly used to designate this disorder and are used interchangeably in current literature. However, since recent ultrastructural studies indicate that the material on the lens capsule is derived, at least in part, from the lens, it is proposed that the disorder be called exfoliation syndrome (XFS).8–10
EPIDEMIOLOGY
Although XFS has been well described in patients of Scandinavian heritage, it also has been documented in numerous populations, including other Europeans,11 the Japanese,12 Australian aborigines,13 Navaho Indians,14, individuals of Mediterranean origin,15 natives of India16 and Pakistan,17 and the southern Bantu tribe of South Africa.18 It is reported, but is relatively rare, among African Americans.19,20
Wide variations in the prevalence of XFS have been reported. These variations may result, at least in part, from differences in the definition of disease, populations studied, age and sex distributions, and examination techniques.21 In one study of 2958 patients older than 60 years of age examined by a single investigator in three countries, Aasved reports respective prevalence rate of exfoliation of 4.0% in England, 4.7% in Germany, and 6.3% in Norway.11 Among Australian aborigines older than 60 years of age, Taylor and coworkers note a 16.3% prevalence rate of exfoliative changes.13 In the United States, a prevalence rate of 1.6% among patients older than 30 years of age and 3.2% for those older than 60 years of age was noted in one study,22 and in another the prevalence rate was 0.7% for those aged 52 to 64, 2.6% for ages 65 to 74, and 5.0% for ages 75 to 85.21
The reported prevalence of increased intraocular pressures and glaucoma in XFS are variable, again depending on the populations evaluated. Kozart and Yanoff report that of 100 consecutive patients with XFS in Philadelphia, glaucoma was present in 7% and ocular hypertension (intraocular pressure above 22 mmHg without optic nerve cupping or visual field loss) was present in 15%.23 According to Henry and colleagues, the cumulative probability of elevated intraocular pressures developing in XFS eyes previously with normal intraocular pressures was 5.3% over 5 years and 15.4% over 10 years.24 Hensen and Sellevold note that glaucoma may develop in 7% to 20% of XFS eyes previously without glaucoma over 5 years and 9% to 24% of these eyes over 10 years.25 The lower figures refer to progression in women and the higher figures to progression in men. These investigators further note that in bilateral XFS and unilateral glaucoma, the second eye developed glaucoma within 5 years in 21% to 26% of cases.
Among patients with open-angle glaucoma, the prevalence rate of XFS varies widely with the geographic location. In the United States, reported figures range from 1% to 12%.9,20,23,26 A prevalence rate of 26% to 75% has been noted in various Scandinavian countries.27–29 Figures from other areas include a 46.9% prevalence rate in eastern Turkey,30 44.5% in northwestern Spain,31 and 1.4% in a South African white population.32
Exfoliation syndrome is predominantly a disorder of the elderly and affects both men and women. Although it has been described in patients as young as 17 years of age,33 it is rare among those younger than 40 years. The reported mean age of onset of this condition ranges from the late 60s to 70s, and the prevalence increases with increasing age.21,34 Although some studies show that exfoliation is more common among men,16,35 several large American and European series indicate that exfoliative changes are more common in women.18,19,34 However, among patients with exfoliative changes, the likelihood of the development of increased intraocular pressures and glaucoma appears to be greater in men.36–38
Some cases of exfoliation may be hereditary. However, no clear inheritance pattern of XFS has been described, and most cases appear to be sporadic.39,40 An epidemiologic study by Ringvold and coworkers reveals a higher than expected prevalence of XFS among both spouses of married couples, suggesting an environmental influence on the distribution of the disorder.41 Ringvold and colleagues also considered an infectious etiology, such as a slow virus, in the pathogenesis of exfoliation syndrome.10,41 Additionally, Küchle and Naumann report the occurrence of exfoliation in three eyes of two young patients 4 to 6 years after penetrating keratoplasty, raising the possibility that XFS is a transmissible disease (from donor buttons to recipients).42
CLINICAL FEATURES
Lens
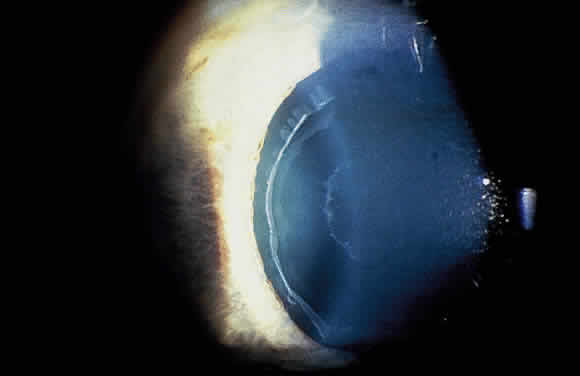
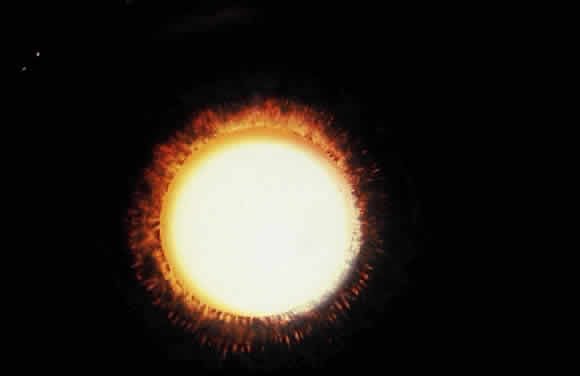
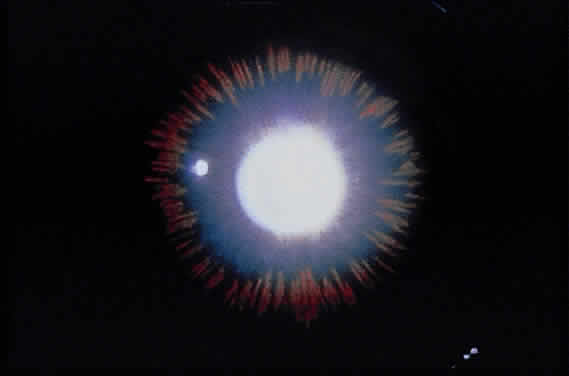
Deposition of exfoliative material on the anterior lens surface is the most commonly recognized feature of XFS and usually is best appreciated after pupillary dilation.43 A bull's-eye pattern generally is seen in which a translucent central zone and a granular peripheral zone of deposition are separated by an intermediate clear zone (Fig. 1). The translucent central zone of exfoliative material varies in diameter and may exhibit curled edges. In approximately 20% of patients with exfoliation, the central zone may be absent.38 The intermediate clear zone presumably results from lens contact with movement of the iris. The granular peripheral zone, which may be subtle but invariably is present in XFS, generally exhibits a well-delineated inner border and often shows numerous radial striations (Fig. 2). Occasionally, a bridge of exfoliative material may cross the clear zone to join the central zone and the granular peripheral zone.38,44
|
|
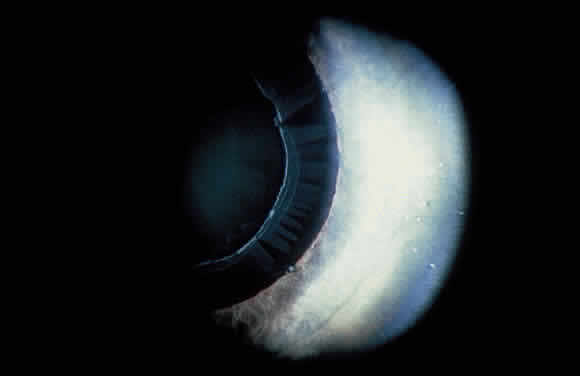
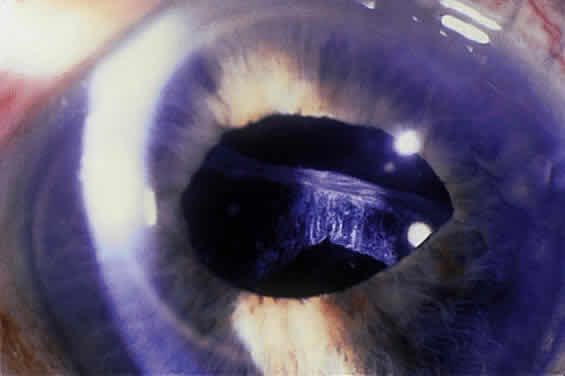
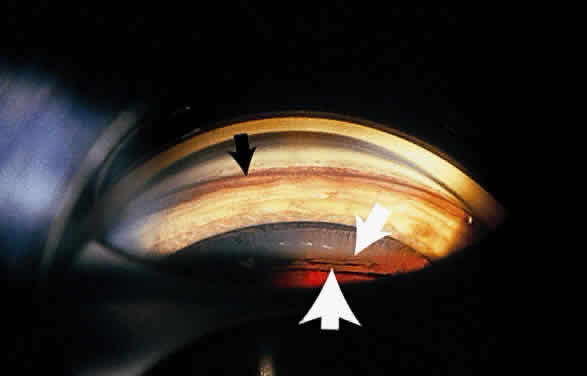
Some patients with XFS exhibit phacodonesis and subluxation of the lens, apparently resulting from degenerative changes in the zonular fibers.45–47 (Fig. 3) Spontaneous dislocation of the lens into the vitreous has been reported.48 In 1970, Bartholomew described 22 spontaneously displaced lenses in 19 patients with the disorder.45 Sixteen of the lenses were subluxated inferiorly. In these eyes, he visualized superior zonular fibers that were coated with exfoliative material and further noted that the zonular breaks generally were present at the ciliary body attachments and not at the lens, leading him to hypothesize that the main degenerative process occurred at the insertion of the zonular fibers to the basement membrane of the ciliary body epithelium. By electron microscopic examination, Schlötzer-Schrehardt and Naumann noted disruption of zonular fiber structure at the ciliary body insertion sites in eyes with XFS but also observed abnormalities at the attachments to the anterior lens capsule.46
|
Bartholomew also describes phacodonesis in XFS and believes that this represents a probable sign of incipient lens displacement.49 On electron microscopic studies, Futa and Furuyoshi demonstrated marked degenerative changes in the lens zonules of XFS eyes with phacodonesis compared with normal zonular structure in those with no phacodonesis.47 Clinically, iridodonesis in association with phacodonesis may not necessarily be observed because of iris infiltration by exfoliative material or, perhaps, prolonged miotic therapy.
Iris and Pupil
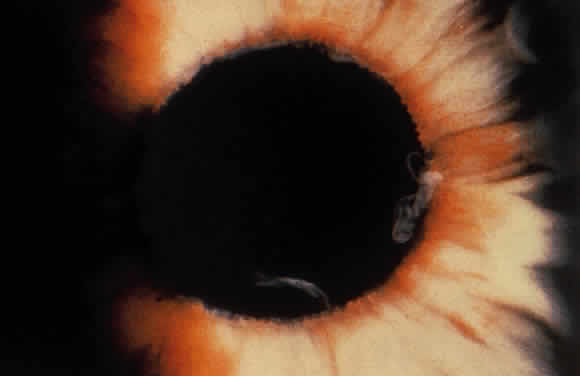
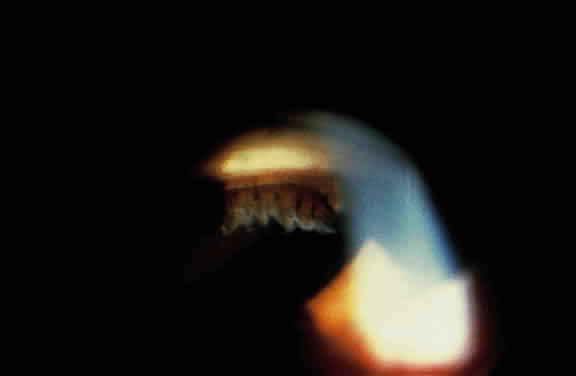
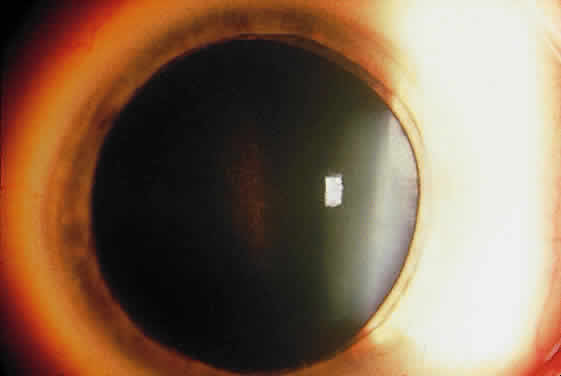
Exfoliative material frequently is observed at the pupillary border (Fig. 4) and should suggest the diagnosis of exfoliation even before the pupil is dilated.27 In addition, defects of the pigmented pupillary ruff are commonly seen. Aasved reports pupillary defects in 6.1% of eyes without XFS and in 74% of eyes with XFS.50 He further notes that in patients with unilateral XFS, pupillary ruff defects were twice as common in the affected eyes when compared with the fellow eyes. Iris transillumination defects in a moth-eaten pattern often are observed. Although these generally are limited to the region of the sphincter (Fig. 5), several patients also have diffuse midperipheral defects.51
|
|
Iris fluorescein angiographic studies in eyes with exfoliation disclose iris hypoperfusion, fewer radial iris vessels, and loss of the normal pattern of these vessels.52 Microneovascularization of the iris and associated fluorescein leakage also have been observed.53 These findings may result from tissue hypoxia caused by abnormal extracellular matrix formation or vascular abnormalities.54,55 Whether tissue hypoperfusion contributes to the development of these abnormalities or is a secondary effect of the XFS is not known. In support of the former are studies that show an increased frequency of XFS in eyes with a history of transient ischemic attacks56,57 and an increased prevalence in patients with a history of vascular disease.58
The iris hypoxia in this disorder may lead to atrophy of the iris pigment epithelium, resulting in particulate pigment deposition on the anterior iris surface seen in some eyes with exfoliation.55 Prince and Ritch note that the pigment often is found in a whorl-like pattern on the sphincter and is most commonly found over the inferior portion of the sphincter, although it also may be diffusely scattered over the peripheral iris surface.43
Typically, the pupil dilates poorly in an exfoliative eye. This likely is partly caused by atrophy and degeneration of the iris muscle cells55,59 and may be another association with iris hypoperfusion. Accumulated stromal iris exfoliative deposits also may contribute to poor mydriasis.
Cornea
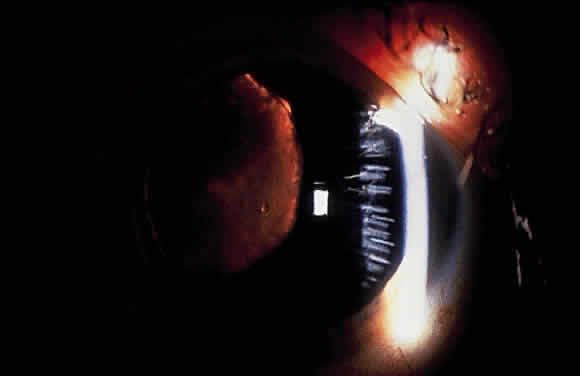
Slit-lamp examination of the cornea may reveal flakes or clumps of exfoliative material deposited on the endothelium43 (Fig. 6). On ultrastructural examination, Schlötzer-Schrehardt and associates note incorporation of the material into Descemet's membrane, leading them to believe that degenerated endothelial cells could be a source of the deposits.60 Pigment on the endothelium also may be observed. It usually is diffuse and unevenly distributed but rarely forms a Krukenberg spindle. Decreased endothelial cell counts and morphologic abnormalities of endothelial cells in eyes with exfoliation, as well as uninvolved fellow eyes of apparent unilateral cases, also have been reported.61–63
Conjunctiva
Clinically, the conjunctiva does not appear to be involved in XFS. However, conjunctival biopsy specimens reveal the presence of exfoliative material,64–67 and fluorescein angiographic studies show loss of the normal limbal vascular patterns, congestion of the anterior ciliary vessels, and possible evidence of neovascularzation.68
Angle and Anterior Chamber
Exfoliation syndrome and pigment dispersion syndrome (PDS) are the two most common causes of increased pigmentation of the trabecular meshwork. The increased trabecular meshwork pigmentation in XFS tends to be less distinct and more patchy than the dense homogeneous deposition of trabecular pigment in PDS43 (Fig. 7). Clinically, the degree of pigmentation in XFS appears to be correlated with the severity of the glaucoma.26,69
Pigment also may be deposited on or anterior to Schwalbe's line (Sampaolesi's line). Occasionally, flakes of exfoliative material may be present in the anterior chamber angle (see Fig. 7). Although the anterior chamber angle usually is open in XFS, acute and chronic angle closure and occludable angles may be seen.9,69–71
Pupillary dilation in XFS may result in marked pigment dispersion into the anterior chamber. This may be accompanied by a substantial increase in the intraocular pressure,43 presumably resulting from decreased aqueous outflow caused by the dispersed pigment. Although pigment dispersion after dilation may occur in patients without XFS, Krause and colleagues have shown that the pigment released after dilation is substantially greater in XFS than in normal persons.72
The dispersed iris pigment on the anterior iris surface, in the angle, and in the anterior chamber after dilation arises from the iris pigment epithelium.73 As mentioned previously, iris hypoxia may result in pigment epithelial atrophy and subsequent pigment dispersion,55 but iris contact with a rough anterior lens surface also may contribute to this phenomenon.
Ciliary Body and Zonules
Exfoliative material also may be seen on the ciliary processes and zonules (Fig. 8). Using a modified method of gonioscopy known as cycloscopy, Mizuno and Muroi documented exfoliative material on the zonules and ciliary processes in all 31 eyes with clinical evidence of exfoliation.12 They further note that in patients with clinically unilateral exfoliation, more than half (17 of 31) of the fellow eyes demonstrated exfoliative material on the ciliary processes and zonules, thus establishing subclinical evidence of exfoliation in clinically “uninvolved” eyes.
|
HISTOPATHOLOGY
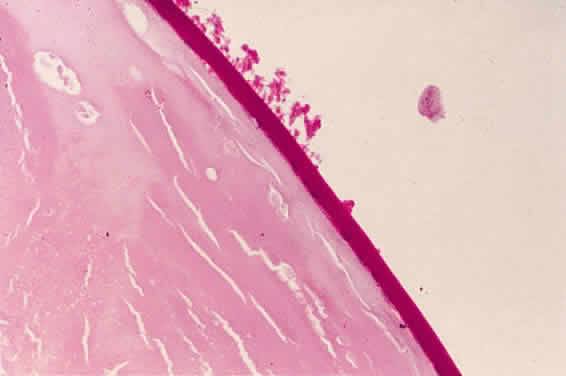
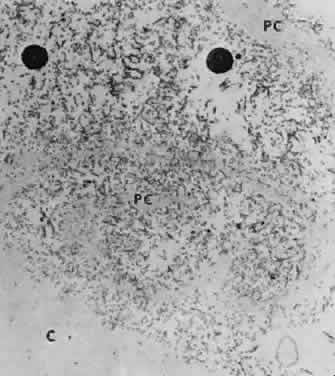
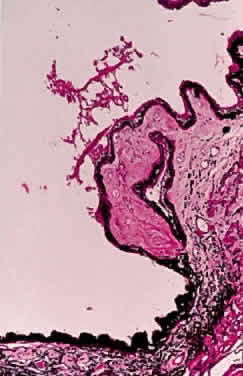
Accumulation of exfoliative material may be detected histopathologically throughout the anterior segment to include the lens, iris, trabecular structures, conjunctiva, ciliary body, and zonules. The deposition of fibrillar eosinophilic material on the anterior lens capsule is the classic histopathologic feature of XFS (Fig. 9). Ultrastructural studies also indicate some degree of actual exfoliative changes or peeling of the anterior lens capsule74 (Figs. 10 and 11). In addition, Ashton and associates describe a degenerative band containing exfoliative material within the inner half of the lens capsule.5 Bertelsen and coworkers independently noted projection of coarse fibrils from the lens surface into the deep portion of the lens capsule to form an amorphous layer of the lens capsule between the lens epithelium and normal lens capsule.6 Thus, studies indicate that exfoliative material accumulates on the anterior lens surface but also that the lens capsule itself may be involved, and that the underlying epithelial layer may at least partially contribute to the production of the exfoliative material.
A ground glass-appearing precapsular layer sometimes is observed on the anterior lens capsule of elderly patients. Ultrastructural studies show this layer to have a fibrillar structure similar to exfoliative material, indicating that this may be a precursor to the XFS.75,76
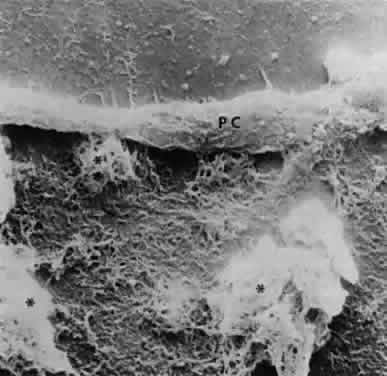
Exfoliative material also may be observed histopathologically on the pupillary border,8 on the anterior iris surface77, within the anterior iris stroma, around iris blood vessels,78–80 and on the posterior surface of the iris.3,4 The iris has been implicated as a source of the material,73,81 and essentially all iris cell types, including epithelial cells, fibrocytes, melanocytes, vascular endothelial cells, pericytes, and smooth muscle cells, may be involved in the production of exfoliative material.55 Exfoliative deposits also have been described on the zonules and ciliary processes (Fig. 12) and within the basement membrane of the nonpigmented ciliary epithelium.4,82 Observations by Mizuno and Muroi suggest that exfoliative material may accumulate on these structures before deposition elsewhere in the eye.12
|
Exfoliative material and pigment have been described in the intertrabecular spaces, trabecular endothelium, juxtacanalicular tissue, and Schlemm's canal.3,4,8,83–85 An association between the extent of trabecular involvement by exfoliative material and intraocular pressure has been noted, with greater involvement producing higher intraocular pressures.86 Although most exfoliative material is carried to the trabeculum by aqueous, the material also may be produced by the trabecular endothelial cells.87,88
Exfoliative material generally is not found in the vitreous or posterior to the hyaloid face. However, it may accumulate on the hyaloid face after intracapsular cataract extraction.44,89
Exfoliative material also may be seen in extrabulbar tissues. As previously noted, exfoliative fibers have been seen in biopsy specimens of conjunctival tissue from eyes with XFS and also from fellow eyes that appeared to be clinically unaffected.64,65 Eagle and colleagues describe similar material in the walls of the short posterior ciliary arteries in the orbit.7 These authors suggest that exfoliative material is an abnormal basement membrane synthesized at multiple sites by aging cells. Histopathologic studies also reveal the material in orbital septa, extraocular muscles, vortex veins, and central retinal vessels.66,67
In 1992, Schlötzer-Schrehardt and associates,90 followed by Streeten and coworkers,91 reported electron microscopic findings of exfoliative fibers in more remote extraocular tissues. They demonstrated the material in lung, heart, liver, gallbladder, skin, kidney, and cerebral meninges of exfoliative patients. It was observed to be within the interstitial fibrovascular connective tissue in close proximity to elastic fibers, collagen fibers, fibroblasts, and vessel walls. This suggests that XFS is a systemic disorder involving generalized abnormal connective tissue metabolism. As noted previously, exfoliation may have an increased prevalence among individuals with systemic vascular conditions.56–58 However, no systemic clinical manifestations resulting from the presence of extraocular exfoliative material have been demonstrated.
NATURE OF EXFOLIATIVE MATERIAL
Exfoliative material consists of an irregular meshwork of fibers composed of fibrillar subunits.74,92 Masses of these fibers correspond to the material seen clinically (see Fig. 10). The individual fibrils, which measure 6 to 8 nm in diameter and exhibit cross-banding at 10- to 12-nm intervals, are hypothesized to consist of macromolecules with a protein backbone and polysaccharide side chains.93–95 In contrast to collagen, the amino acid content of exfoliative material lacks hydroxyproline.96 Ringvold and Husby noted histochemical, immunologic, and ultrastructural features that led them to classify exfoliative material as an amyloid-like substance.97 Repo and associates note Congo red-positive staining for amyloid in the vessel walls of 7 of 13 iris specimens from exfoliation patients and believe that these findings support the theory that XFS is associated with amyloid.59 Immunohistochemical studies by other investigators show heparin sulfate and chondroitin sulfate proteoglycans, laminin, entactin/nidogen, fibronectin, and amyloid P protein to be components of exfoliation material.98,99 Schlötzer-Schrehardt and coworkers suggest that the material may be an expression of a disordered extracellular matrix synthesis.98
As previously noted, Eagle and associates regarded exfoliative material as abnormal basement membrane material.7 In support of this assumption, other studies indicate that it may be a basement membrane proteoglycan.98,100 Streeten and coworkers report that, like zonular fibers, exfoliative material demonstrates staining characteristics similar to oxytalan, the microfibrillar component of elastic fibers.101 These investigators also have shown an immunologic relation to elastic microfibrils and elastin.95,102 The association of exfoliative material with elastosis in conjunctival biopsy specimens from XFS patients offers some support for the hypothesis that exfoliation represents a type of elastosis.103 In this elastosis, basement membrane-secreting cells appear to produce an abnormal protein-mucosubstance complex with elastic microfibrillar characteristics.
DIFFERENTIAL DIAGNOSIS
Pigment dispersion syndrome and true exfoliation of the lens capsule are included in the differential diagnosis of XFS. In contrast to the elderly population affected by XFS, PDS and, more specifically, pigmentary glaucoma tend to occur bilaterally in the eyes of young myopic patients.104 Peripheral radial iris transillumination defects are seen rather than the peripupillary atrophy of XFS. In addition, Krukenberg spindle generally is seen in pigmentary glaucoma, and the trabecular pigmentation in pigmentary glaucoma tends to be more dense and homogeneous than the patchy trabecular hyperpigmentation in XFS. The characteristic appearance of exfoliative material on the anterior lens capsule is not present in pigmentary glaucoma. However, XFS may occur in patients with PDS.105
In true exfoliation of the lens capsule secondary to intense heat,106 inflammation,107 trauma,107,108 or irradiation,107 the lens capsule is rolled up in characteristic clear, thin sheets. This condition, which also may be called capsular delamination,109 is only infrequently associated with glaucoma. Histopathologically, a prominent split in the anterior lens capsule is seen without associated deposition of exfoliative material.
LATERALITY
Clinically evident exfoliative changes initially are unilateral in a large percentage of patients with XFS, although these changes frequently become bilateral over time. In their series of 100 consecutive patients, Kozart and Yanoff describe 76 patients with unilateral pseudoexfoliation and 24 with bilateral changes.23 In patients who initially presented with unilateral exfoliation, Hansen and Sellevold noted the development of exfoliation in the fellow eyes in 31% of women and 40% of men over 5 years.25 Aasved describes similar progression in 43% of patients over 6 to 7 years.110 Henry and coworkers report a 5-year probability of 16.8% for developing XFS in the fellow eyes of initially unilateral patients.24 Patients with unilateral exfoliation tend to be younger than patients with bilateral exfoliation.37,38,44
These probabilities refer to the development of clinically evident exfoliation in fellow eyes of unilateral patients. As emphasized by Mizuno and Muroi, many, if not most, of fellow eyes in patients with clinically “unilateral” XFS may have early subclinical exfoliation.12 Results of conjunctival biopsies in fellow eyes of clinically unilateral patients that showed deposition of exfoliative material also support this conclusion.64,65
EXFOLIATION SYNDROME AND GLAUCOMA
When exfoliative changes are accompanied by glaucoma, the term exfoliative (or pseudoexfoliative) glaucoma is most commonly used. In eyes that develop exfoliative glaucoma, intraocular pressures generally are higher than in eyes with primary open-angle glaucoma.111–114 Presumably because of these higher intraocular pressures, optic nerve damage and visual field loss generally are greater in XFS when compared with primary open-angle glaucoma.87,115,116 Although a recent study by Jonas and Papastathopoulos found no significant variation in the disc appearance when they compared these two groups,117 comparative optic disc analysis performed by Tezel and Tezel reveals more diffuse neuroretinal rim damage in glaucomatous exfoliative eyes compared with more sectoral damage in primary open-angle glaucoma patients.118 Elastosis of the lamina cribrosa has been noted on electron microscopic studies in exfoliative eyes, suggesting that abnormal elastin may be a feature of the optic nerve composition in this disorder.119
The medical treatment of exfoliative glaucoma is similar to that for primary open-angle glaucoma and includes the use of topical β-blockers, alpha-adrenergic agonists, prostaglandin analogues, and topical or oral carbonic anhydrase inhibitors. Cholinergic agents and epinephrine compounds are less frequently used, since the introduction of newer ocular antihypertensives but represent additional medical options. The glaucoma in XFS tends to be less responsive to medical therapy than in primary open-angle glaucoma,9,115,120–123 and a higher percentage of XFS patients requires surgical intervention.110,114,115,120
Unlike patients with primary open-angle glaucoma, those with XFS respond to topical corticosteroids in a manner similar to normal persons.124,125 In a study of corticosteroid responsiveness, Pohjola and Horsmanheimo report that 11 of 39 XFS patients (28%) experienced rises in intraocular pressure of more than 6 mmHg after 4 weeks of treatment.124 This markedly contrasts to a much higher intraocular pressure response rate (above 90%) in patients with primary open-angle glaucoma.126–128
Eyes with XFS generally are agreed to be at risk for developing glaucoma and eyes with greater clinical evidence of XFS usually demonstrate higher intraocular pressures than eyes with lesser degrees of involvement. In a patient with unilateral exfoliation, for instance, the intraocular pressure generally is higher in the involved eye when compared with the uninvolved eye. Indeed, exfoliation should be strongly considered in the differential diagnosis of an elderly patient who presents with unilateral elevation of intraocular pressure.
Exfoliative glaucoma is considered a secondary glaucoma, since the accumulation of exfoliative material and pigment within the trabecular structures84,129,130 generally is believed to be associated with obstruction of aqueous outflow and a rise in intraocular pressure. That exfoliative glaucoma is a secondary rather than a primary glaucoma also is supported by the relative lack of intraocular pressure responsiveness to topical corticosteroids.124,125 However, many eyes with pigment dispersion and clinically evident exfoliation have normal intraocular pressures and do not develop glaucoma.24 Furthermore, some patients with clinically unilateral exfoliation have bilaterally elevated intraocular pressures. These findings suggest that, at least in some individuals, an underlying dysfunction of the trabecular meshwork may accompany exfoliative glaucoma,131 or that an underlying primary open-angle glaucoma also is present.
As described elsewhere, exfoliative glaucoma sometimes is associated with angle closure mechanism.9,69,71 Also notice that patients with exfoliation sometimes present with an acute and significant intraocular pressure rise, even in the presence of an open angle.
RESPONSE OF EXFOLIATIVE GLAUCOMA TO LASER AND INCISIONAL SURGICAL THERAPY
Because XFS patients are less responsive to medical treatment, surgical intervention often is required. Argon laser trabeculoplasty initially is effective in decreasing intraocular pressure in XFS.132–134 In one study of 34 XFS patients, the average intraocular pressure after argon laser trabeculoplasty fell from 28.5 to 16.1 mmHg, with an average follow-up of 5 months.134 Loss of effect with long-term follow-up has been described, sometimes with relative abrupt and unexpected rises in intraocular pressure.132
Glaucoma filtering surgery in exfoliative glaucoma appears to be at least as successful as that performed in eyes with primary open-angle glaucoma. Jerndal and Kriisa report that trabeculectomy (performed without use of adjunctive antifibrotics) effectively lowered intraocular pressure to less than 20 mmHg without medications in 42 of 52 patients (81%) with follow-up greater than 6 months.135 A prospective study by Konstas and associates shows a higher success rate after trabeculectomy among exfoliative glaucoma eyes (mean untreated postoperative intraocular pressure of 11.8 ± 4.4 mmHg) compared with the response in those with primary open-angle glaucoma (mean untreated postoperative intraocular pressure of 15.0 ± 4.6 mmHg) at 6 months of follow-up.114
Some observers note that intraocular pressures may drop after removal of the crystalline lens in eyes with XFS.136–138 It was originally hypothesized that removal of the lens also removed the source of exfoliative material,136,137 which is known to be untrue.89 Although lens removal often is necessary in patients with coexisting cataract and glaucoma, lens removal alone is not a recommended treatment for exfoliative glaucoma.
A new modality involving trabecular aspiration, in which trabecular debris and pigment are cleared from the anterior chamber angle of eyes with XFS to achieve improved aqueous outflow, has been reported by Jacobi and coworkers.139,140 In their most recent report, they observed that 54% of exfoliative patients who underwent the procedure in combination with cataract extraction and 45% of patients who underwent only the aspiration procedure had adequate control of intraocular pressures with no glaucoma medical therapy after 2 years of follow-up.140 They did note loss of response to the surgery over time, however, presumably because of reaccumulation of exfoliative material in the trabecular meshwork.
EXFOLIATION SYNDROME AND ANGLE-CLOSURE GLAUCOMA
Exfoliation generally is viewed as a secondary open-angle glaucoma. Bartholomew reports normal anterior chamber depths in XFS.141 However, Layden and Shaffer report a 23% prevalence rate of anterior chamber angles of grade II or less among patients with XFS and also report acute angle closure in four patients.9 Wishart and associates have recently described the presence of peripheral anterior synechiae in 14% of XFS patients and occludable angles in an additional 18%,69 and Gross and coworkers note occludable angles (but no acute angle-closure episodes) in 9.3% of exfoliative eyes.71
Dark proposes that angle closure in XFS may result from the development of iridocapsular adhesions and subsequent pupillary block.142 Other possible mechanisms of angle closure include anterior lens movement resulting from weak zonules and prolonged miotic therapy with forward displacement of the lens-iris diaphragm.143
EXFOLIATION SYNDROME, CATARACTS, AND CATARACT SURGERY
An increased prevalence of cataracts has been described in patients with XFS.21,26 It is unlikely that the exfoliation predisposes to cataract formation. Rather, both XFS and cataracts are conditions of the elderly that may coexist.
Problems during lens extractions in patients with XFS have been well described in the literature. Early reports note an increased incidence of intraoperative lens dislocation, vitreous loss, and capsular rupture in eyes with XFS undergoing intracapsular cataract extraction.144–146 Dark observes that synechia formation between the iris pigment epithelium and the peripheral anterior lens capsule can lead to capsular rupture during the intracapsular procedure.142 With extracapsular cataract extraction, several surgeons report an increased risk of zonular and capsular breaks in exfoliation eyes143,147–150 (Fig. 13). Guzek and coworkers, for example, report that the risk of zonular breaks in 241 patients with XFS during extracapsular cataract extraction was four times greater than in 736 non-XFS patients.148 Similarly, an increased frequency of intraoperative complications has been observed in exfoliative eyes undergoing phacoemulsification. Scorolli and associates found the risk of complications to be about five times greater when XFS was present.151 Droslum and coworkers note a 9.6% incidence rate of capsular tears, zonular tears, or vitreous loss during phacoemulsification procedures in XFS eyes compared with a 3.7% rate of the same complications in non-XFS cases.152
The increased frequency of zonular breaks in exfoliative patients undergoing cataract procedures is believed to be primarily caused by degeneration of the zonular fibers.47,153 Other factors contributing to complicated cataract surgery in eyes with XFS include poor pupillary dilation150,153,154 and, as noted previously, synechiae between the iris pigment epithelium and anterior lens capsule.142
Intraoperative and postoperative posterior chamber intraocular lens implant subluxation also has been reported in association with XFS.155,156 Placement of the posterior chamber lens haptics into the ciliary sulcus rather than the capsular bag in XFS may enhance stability of the implant.148,156 Although the lens implant generally is well tolerated in XFS eyes that have undergone cataract extraction, an increased incidence of early postoperative fibrinoid iritis in these eyes has been reported.149,157 Zetterstrom and coworkers note a reduction of this iritis when heparin surface modified intraocular lenses are implanted.158