Blunt trauma to the globe typically compresses the cornea, shortening the globe along its anterior-posterior axis. The fluid and other intraocular contents are relatively noncompressable, and the globe deforms with elongation in the equatorial plane. This stretching may lead to rupture of one or more of the seven relatively nondistensible rings within the eye: the pupillary sphincter (radial tears), the iris insertion (iridodialysis), the ciliary body (angle recession) and its attachment to the sclera (cyclodialysis), the trabecular meshwork (trabeculodialysis), the zonular fibers, and the ora serrata (retinal dialysis). Damage to these structures leads to many of the various forms of glaucoma following trauma.
HYPHEMA
Immediately following blunt and penetrating trauma, damage to the iris and ciliary body compromises the blood aqueous barrier, with the release of protein into the anterior chamber, or actual bleeding. Protein and blood cells may obstruct the trabecular meshwork, thus increasing IOP.1–3
Inflammation following trauma is typically treated with topical steroids. Cycloplegics are helpful to reduce discomfort, to stabilize the blood-aqueous barrier, and to enhance uveoscleral outflow. This aids IOP control and helps prevent posterior synechiae formation and resultant pupillary block. Cycloplegics may also help prevent rebleeds following hyphemas by immobilizing the iris and ciliary body.
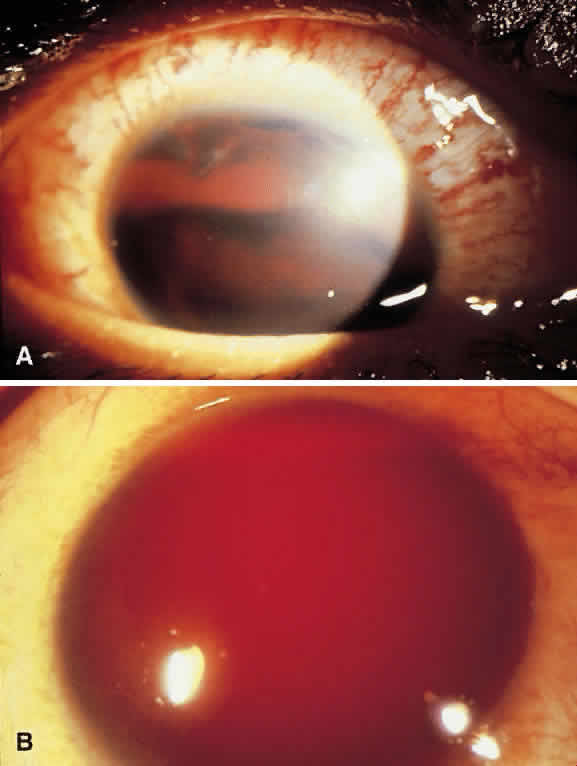
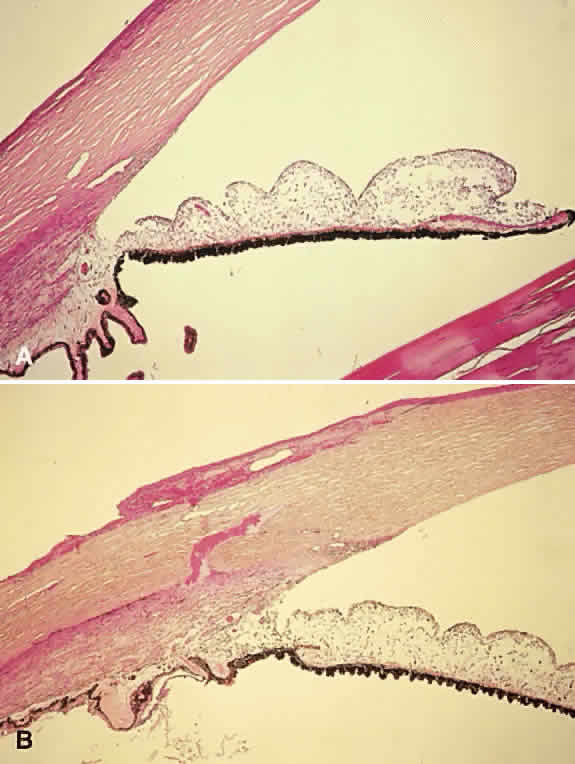
Hyphema is a relatively common complication of ocular trauma (Fig. 1). Bleeding commonly results from tears between the circular and longitudinal muscles of the ciliary body, disrupting the major arterial circle of the iris. These tears produce angle recession. Hyphemas frequently lead to elevated IOP; the larger the hyphema the greater is the likelihood of pressure elevation. The mechanism of glaucoma is obstruction of the trabecular meshwork by trabecular beam swelling, fibrin, and red blood cells. Because of their pliability, red blood cells, in small amounts, pass through normal trabecular meshwork with relative ease.1 However, in larger quantities, especially when the accompanied by fibrin and trabecular swelling, red blood cells may obstruct the meshwork.1,2 Microhyphemas, in which the suspended red blood cells do not form an observable layer, also may lead to elevated IOP. Glaucoma occurs in over 25% of hyphemas filling 50% of the anterior chamber, over 50% of near total hyphemas, and in almost all “black ball” or “eight ball” hyphemas.
|
In a “black ball” hyphema, the blood is so concentrated and the anterior chamber so stagnant and hypoxic, that the blood within the anterior chamber appears black (Fig. 2). Such a black ball hyphema may consist of a dumbbell shaped clot filling the anterior chamber, pupil, and posterior chamber. These hyphemas are more difficult to manage and lead to glaucoma through pupillary block as well as trabecular obstruction.4
|
Optimal treatment for hyphema has not been established. Most clinicians recommend treatment with topical steroids and cycloplegics. Patients are often instructed to keep their heads elevated to promote settling of the red blood cells in the inferior anterior chamber angle, thus improving vision and allowing more of the trabecular meshwork to clear. Bed rest and the avoidance of nonsteroidal anti-inflammatories or other agents with blood thinning properties are recommended to reduce the incidence of rebleeds, which commonly occur in the first 4 days.
Rebleeds often are more severe than the initial bleeding episode and may lead to more serious complications and vision loss. Oral aminocaproic acid and steroids have been shown to reduce the rate of rebleeds. Oral aminocaproic acid, however, may cause nausea, vomiting, and systemic hypotension; thus, its use typically requires inpatient hospitalization. Topical aminocaproic acid may also reduce rebleeding.5 Most hyphemas can be managed on an outpatient basis unless the patient is at high risk of rebleeding and complications.6,7 Such patients include those receiving anticoagulant therapy, children, African Americans, patients who tested positive for sickle cell (including trait), patients with greater than one-third hyphemas, and patients at high risk for noncompliance with therapy or follow-up.
Aqueous suppressants are helpful to control elevated IOP. Miotics are avoided because they may increase inflammation or rebleeding. Latanoprost should also be avoided, given concerns regarding increased inflammation and current lack of evidence that it is effective in this setting. Most patients without pre-existing nerve damage or sickle cell disease or trait may be observed, despite moderately elevated IOPs (up to 35 to 40 mm Hg) for 2 to 4 weeks, with only conservative medical therapy.
Patients with severely or persistently elevated pressures may be treated with paracentesis, anterior chamber washout, clot extraction (either manual or facilitated by a mechanical vitrector), or guarded filtration procedures. Some clinicians routinely perform a guarded filtration procedure at the time of anterior chamber washout to help ensure short-term IOP control. The expectation with this combined procedure is that the filtration surgery will most likely fail in the intermediate future, by which time the trabecular meshwork should have recovered adequate function. Trabeculectomy with iridectomy has been recommended as the initial treatment for total hyphemas, because most require surgery eventually.8 During anterior chamber washout, it is not necessary to remove all of the clot, because it is the circulating red blood cells that obstruct the meshwork, and clot removal may lead to rebleeding and damage to intraocular structures. When necessary, an automated vitrectomy cutting handpiece with irrigating sleeve can be helpful to debulk clots without disturbing the structures to which the clots are attached. Ideally, if the clinical situation allows, surgical procedures should be done on or after the fourth day following the trauma, because the highest incidence of rebleeding is between the second and fourth days. Additionally, by this time, clots may have retracted from adjacent structures, facilitating their removal with reduced surgical manipulation and trauma.
All African-American patients or patients with a family history of sickle cell trait or disease who present with a hyphema or microhyphema must be screened for sickle cell trait. The sickle cell gene is present in approximately 9% of African Americans. In the setting of a hyphema, patients with sickle cell trait alone or sickle cell disease may have clinically significant sickling of red blood cells in the relatively hypoxic and low pH conditions of the anterior chamber. Sickled red blood cells are less pliable and easily obstruct the trabecular meshwork, leading to high IOPs even with remarkably little blood in the anterior chamber.9 In addition, patients with sickle cell trait or disease are at risk of ischemic complications to the optic nerve or retina at relatively low IOPs.10,11 For this reason, it has been suggested that sickle patients with hyphemas or microhyphemas be monitored more closely, and that their IOP be managed aggressively to be maintained below 30 mm Hg at all times, and not be allowed to remain higher than 24 mm Hg for more than 24 hours.
Therapies for elevated IOP secondary to hyphemas in patients with sickle cell disease are similar to those in patients without sickle cell. However, there are some important additional considerations.12 Carbonic anhydrase inhibitors may lead to systemic acidosis, potentially precipitating systemic sickle crises or exacerbating sickling in the anterior chamber with attendant complications. Methazolamide has less potential for this than acetazolamide. The significance of the effects of topical carbonic anhydrase inhibitors on the aqueous in this setting is unknown. Systemic osmotic agents may dehydrate patients and also promote systemic sickling in those so predisposed. Epinephrine may increase anterior chamber acidosis through its ischemic effects; the potential of apraclonidine to do the same is unproven. Brimonidine's effects in this situation have also not been fully described. However, brimonidine is highly selective for α-2 (1800:1 α-2:α-1) thus it has less vasoconstrictive (α-1 mediated) effects compared with the other α agonists. Earlier paracentesis has been recommended in the presence of sickle cell trait or disease.12
Corneal blood staining, in which hemoglobin enters the cornea stroma, is another potential complication of hyphemas. The likelihood of corneal blood staining is correlated with longer duration of blood in the anterior chamber, higher IOP, and reduction in the health of the corneal endothelium.13,14 Corneal blood staining clears eventually, starting peripherally, unless the staining is severe or the cornea has underlying dysfunction. Complete clearing may take 6 months or more. In children under 9 years of age, a dense amblyopia may result. For this reason, persistent hyphemas should be more aggressively managed in pediatric patients.
LENS-RELATED GLAUCOMAS
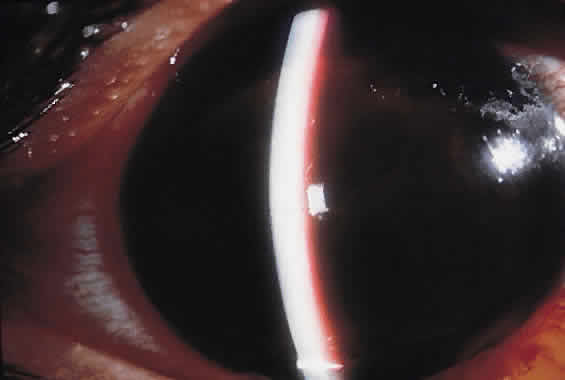
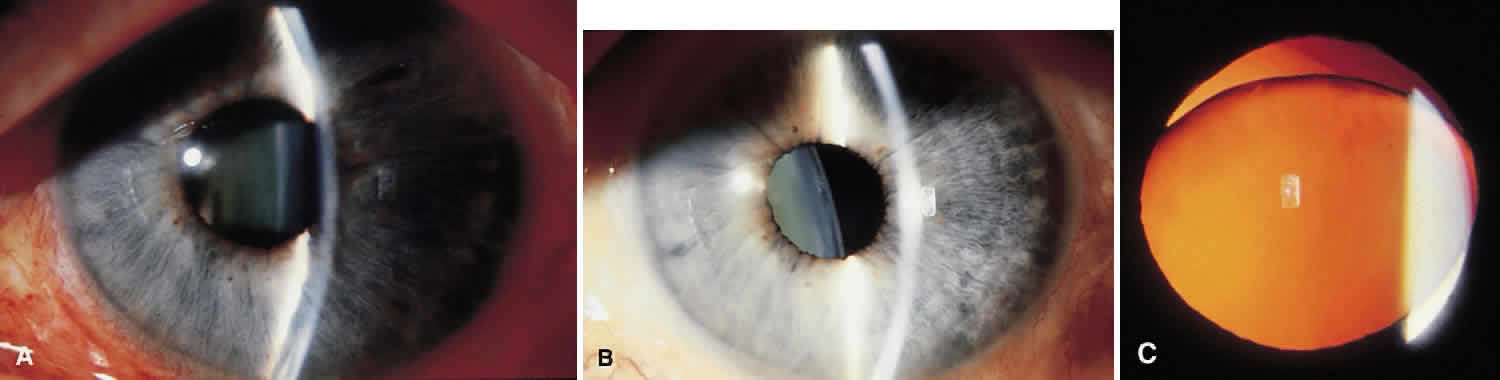
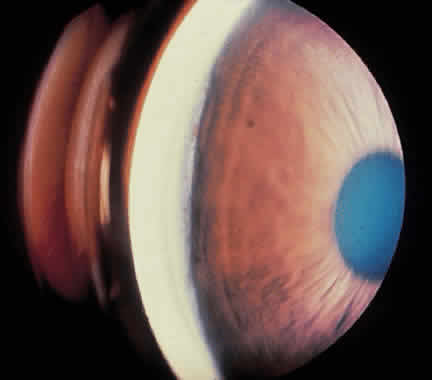
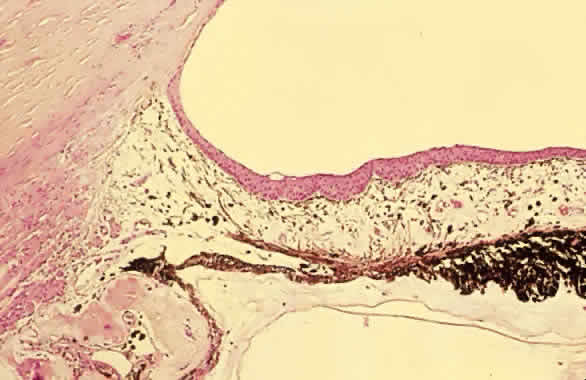
Traumatic dislocation or subluxation of the crystalline lens may lead to pupillary block glaucoma. The loose lens may be free to move anteriorly into the pupil or anterior chamber intermittently, which creates pupillary block (Fig. 3). The loose lens may also produce acute and chronic angle closure by a mass effect, pushing the iris anteriorly. A significantly dislocated lens may allow the vitreous to come forward and cause pupillary block glaucoma. Assessment of lens motility is important in planning glaucoma surgery for any patient with a history of trauma. Occasionally, a lens is noted to be loose at the beginning of surgery, when the supine position leads to the lens's drifting posteriorly or allows the vitreous to come forward around the lens. Remarkably, the lens often re-establishes its original position when the patient is again upright following surgery. Vitreous in the anterior chamber may block the sclerostomy, thus leading to filtration failure.
Phacolytic glaucoma may rarely occur following blunt trauma. Significant contusion injury to the lens may lead to massive swelling of the lens and leakage of lens proteins. Aqueous outflow is obstructed by these proteins and by macrophages that engulf them.3 Swollen lenses may also lead to phacomorphic glaucoma with angle closure. Lens particle glaucoma may occur when the lens capsule is torn by sharp, or occasionally by blunt, trauma lead to trabecular meshwork obstruction by lens fragments.
CHEMICAL INJURIES
Exposure to strongly basic solutions may denature the proteins in the sclera and lead to scleral shrinkage with an acute rise in IOP. Such pressure may drop transiently, only to elevate severely once again following release of prostaglandins.15,16 In rabbits, this secondary pressure rise was blocked by therapy with oral aspirin or indomethacin.16 These glaucomas are treated with aqueous suppressants and topical steroids. However, associated severe corneal injuries, which often present the risk of full thickness corneal melts, may limit the use of steroids.
CAROTID CAVERNOUS AND DURAL FISTULAS
Trauma may tear the internal carotid artery within the cavernous sinus, elevating the local venous system pressure to arterial pressure level.17,18 Tears in the branches of the internal and external carotid arteries may lead to communications with the dural sinus. A sign of this is the presence of dilated vessels beneath the conjunctiva, especially when these do not blanch with epinephrine exposure. Blood may be seen in Schlemm's canal on gonioscopy. Superior vena cava syndrome and thrombosis of an orbital vein or of the cavernous sinus can produce similar findings.
The aqueous outflow pathway may be summarized as follows: anterior chamber → Schlemm's canal → aqueous veins → episcleral veins → anterior ciliary veins → superior ophthalmic vein → cavernous sinus → internal jugular vein. Some conjunctival veins also carry aqueous through the facial vein to the external jugular vein.
Goldmann's equation, which describes the relation of IOP to aqueous flow and episcleral venous pressure, is Po = F/C + Pev; where Po is IOP, F is aqueous production, C outflow facility, and Pev is episcleral venous pressure. This theoretical model has shown good correlation with clinical experience, animal research, and human cadaver experiments. The equation predicts that increases in episcleral venous pressure lead directly to increases in IOP. Normal episcleral venous pressure is between 9 and 10 mm Hg.
Arterialization of the outflow pathways of the eye also may lead to ocular ischemia and neovascular glaucoma.19 Increased venous pressure may lead to choroidal effusions and suprachoroidal hemorrhages with shallowing of the anterior chamber and secondary angle closure glaucoma. Because the mechanism of the angle closure glaucoma is not pupillary block, iridectomy is not helpful.
Treatment of the underlying cause of increased venous pressure is ideal but not always possible. Embolization of carotid-cavernous fistulas carries significant risk of central nervous system vascular accident; many dural fistulas close spontaneously. Aqueous suppressants often are helpful in controlling IOP. Miotics and argon laser trabeculoplasty are not useful given the typically normal facility of outflow. Guarded filtration surgery (trabeculectomy) is effective in that it bypasses the elevated episcleral venous pressure, but it has a higher incidence in these patients of serous and hemorrhagic choroidal detachments. Tight closure of the scleral flap covering the entrance to the anterior chamber is recommended to reduce the incidence of serous and hemorrhagic choroidal effusions. Postoperative suture release can then be used to titrate the reduction in IOP, minimizing sudden pressure changes and the potential for choroidal hemorrhage and effusions. Some clinicians advocate prophylactic “scleral windows” over the ciliary body, either as flaps or by thinning the sclera to about one half its normal thickness, to facilitate drainage of choroidals and prevent large or expulsive choroidal detachments. These are typically placed in the inferior quadrants.