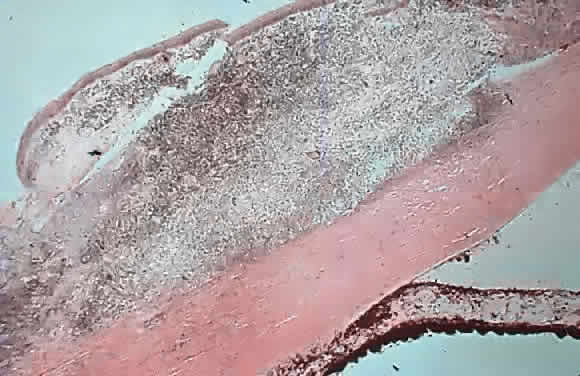
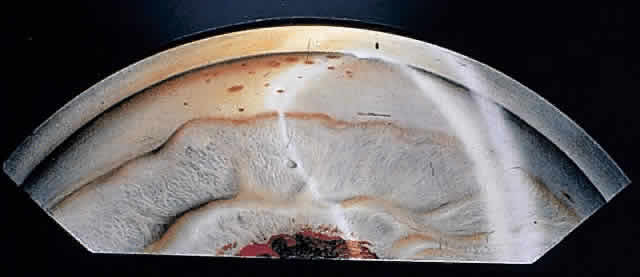
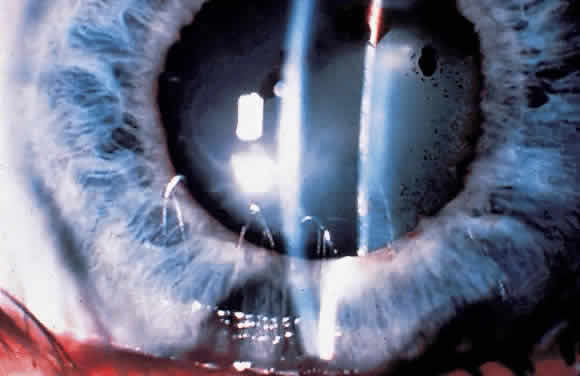
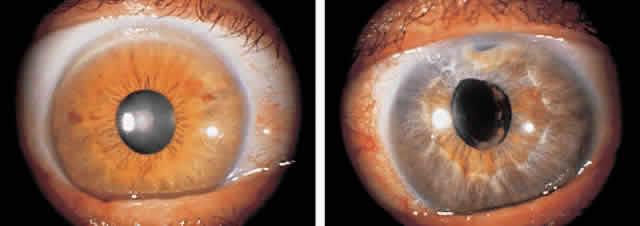
1. Pederson JE: Ocular hypotony. Trans Ophthalmol Soc UK 105:220–226, 1986 2. Ritch R: Pathophysiology of glaucoma in uveitis. Trans Ophthalmol Soc UK 101:321–324, 1981 3. Merayo-Lloves J, Power WJ, Rodriguez A et al: Secondary glaucoma in patients with uveitis. Ophthalmologica 213:300–304, 1999 4. Kanski JJ, Shun-Shin GA: Systemic uveitis syndromes in childhood: an analysis of 340 cases. Ophthalmology 91:1247–2352, 1984 5. Panek WC, Holland GN, Lee DA et al: Glaucoma in patients with uveitis. Br J Ophthalmol 74:223–227, 1990 6. Cabral DA, Petty RE, Malleson PN et al: Visual prognosis in children with chronic anterior uveitis and arthritis.J Rheumatol 21:2370–2375, 1994 7. Lyons JL, Rosenbaum JT: Uveitis associated with inflammatory bowel disease compared with uveitis
associated with spondyloarthropathy. Arch Ophthalmol 115:61–64, 1997 8. Wolf MD, Lichter PR, Ragsdale CG: Prognostic factors in the uveitis of juvenile rheumatoid arthritis. Ophthalmology 94:1242–1248, 1987 9. Falcon MG, Williams HP: Herpes simplex kerato-uveitis and glaucoma. Trans Ophthalmol Soc UK 98:101–104, 1978 10. Womack LW, Liesegang TJ: Complications of herpes zoster ophthalmicus. Arch Ophthalmol 101:42–45, 1983 11. Boniuk M: Glaucoma in the congenital rubella syndrome. Int Ophthalmol Clin 12:121–136, 1972 12. Malla OK, Brandt F, Anten JG: Ocular findings in leprosy patients in an institution in Nepal (Khokana). Br J Ophthalmol 65:226–230, 1981 13. Shields JA, Waring GOd, Monte LG: Ocular findings in leprosy. Am J Ophthalmol 77:880–890, 1974 14. Merritt JC, Callender CO: Adult cytomegalic inclusion retinitis. Ann Ophthalmol 10:1059–1063, 1978 15. Shields JA: Ocular toxocariasis: A review. Surv Ophthalmol 28:361–381, 1984 16. Jensen AD, Naidoff MA: Bilateral meningococcal endophthalmitis. Arch Ophthalmol 90:396–398, 1973 17. Riffenburgh R: Iritis and glaucoma associated with mumps. Arch Ophthalmol 51:1954 18. Saari KM: Acute glaucoma in hemorrhagic fever with renal syndrome (nephropathia epidemica). Am J Ophthalmol 81:455–461, 1976 19. Ben-Sira I, Yassur Y: Ocular onchocerciasis in Malawi: A comparative study of 500 patients and 500 controls. Br J Ophthalmol 56:617–620, 1972 20. Crawford JB: Toxoplasma retinochoroiditis. Arch Ophthalmol 76:829–832, 1966 21. Pettit TH, Learn RN, Foos RY: Intraocular coccidioidomycosis. Arch Ophthalmol 77:655–661, 1967 22. Liesegang TJ: Clinical features and prognosis in Fuchs' uveitis syndrome. Arch Ophthalmol 100:1622–1626, 1982 23. Posner A, Scholssman A: Syndrome of unilateral recurrent attacks of glaucoma with cyclitic symptoms. Arch Ophthalmol 39:517, 1948 24. Chan CC, Roberge RG, Whitcup SM et al: 32 cases of sympathetic ophthalmia: A retrospective study at the National
Eye Institute, Bethesda, MD, from 1982 to 1992 [published erratum
appears in Arch Ophthalmol 1995 Dec;113(12):1507]. Arch Ophthalmol 113:597–600, 1995 25. Henderly DE, Genstler AJ, Rao NA et al: Pars planitis. Trans Ophthalmol Soc UK 105:227–232, 1986 26. Filipe JC, Palmares J, Delgado L et al: Phacolytic glaucoma and lens-induced uveitis. Int Ophthalmol 17:289–293, 1993 27. Sainz de la Maza M, Jabbur NS, Foster CS: Severity of scleritis and episcleritis. Ophthalmology 101:389–396, 1994 28. Lichter PR, Shaffer RN: Interstitial keratitis and glaucoma. Am J Ophthalmol 68:241–248, 1969 29. Cohen RG, Wu HK, Schuman JS: Glaucoma with inflammatory precipitates on the trabecular meshwork: A report
of Grant's syndrome with ultrasound biomicroscopy of precipitates. J Glaucoma 5:266–270, 1996 30. Bill A: Editorial: The drainage of aqueous humor. Invest Ophthalmol 14:1–3, 1975 31. Wilhelmus KR, Grierson I, Watson PG: Histopathologic and clinical associations of scleritis and glaucoma. Am J Ophthalmol 91:697–705, 1981 32. Hogan M, Kimura S, Thygeson P: Pathology of herpes simplex kerato-iritis. Am J Ophthalmol 57:551, 1964 33. Epstein DL, Hashimoto JM, Grant WM: Serum obstruction of aqueous outflow in enucleated eyes. Am J Ophthalmol 86:101–105, 1978 34. Knox D: Glaucoma following syphilitic interstitial keratitis. Arch Ophthalmol 66:44, 1961 35. Rohen JW, Zypen EVd: The phagocytic activity of the trabecular meshwork endothelium: An electron-microscopic
study of the vervet (Cercopithecus aethiops). Graefes Arch Clin Exp Ophthalmol 175:143–160, 1968 36. Richardson TM, Hutchinson BT, Grant WM: The outflow tract in pigmentary glaucoma: a light and electron microscopic
study. Arch Ophthalmol 95:1015–1025, 1977 37. Watson PG, Hayreh SS: Scleritis and episcleritis. Br J Ophthalmol 60:163–191, 1976 38. Waitzman MB, King CD: Prostaglandin influences on IOP and pupil size. Am J Physiol 212:329–334, 1967 39. Eakins KE, Whitelocke RA, Bennett A et al: Prostaglandin-like activity in ocular inflammation. Br Med J 3:452–453, 1972 40. Masuda K, Izawa Y, Michima S: Prostaglandins and glaucomato-cyclitic crisis. Jpn J Ophthalmol 19:368–375, 1975 41. Quinlan MP, Hitchings RA: Angle-closure glaucoma secondary to posterior scleritis. Br J Ophthalmol 62:330–335, 1978 42. Phelps CD: Angle-closure glaucoma secondary to ciliary body swelling. Arch Ophthalmol 92:287–290, 1974 43. Kimura SJ, Hogan MJ, O'Connor GR et al: Uveitis and joint diseases. Arch Ophthalmol 77:309–316, 1967 44. Mapstone R, Woodrow JC: HLA27 and acute anterior uveitis. Br J Ophthalmol 59:270–275, 1975 45. Brewerton DA, Caffrey M, Nicholls A et al: Acute anterior uveitis and HLA 27. Lancet 2:994–996, 1973 46. Rothova A, van Veenedaal WG, Linssen A et al: Clinical features of acute anterior uveitis. Am J Ophthalmol 103:137–145, 1987 47. Moorthy RS, Mermoud A, Baerveldt G et al: Glaucoma associated with uveitis. Surv Ophthalmol 41:361–394, 1997 48. Tay-Kearney ML, Schwam BL, Lowder C et al: Clinical features and associated systemic diseases of HLA-B27 uveitis. Am J Ophthalmol 121:47–56, 1996 49. Linssen A, Meenken C: Outcomes of HLA-B27-positive and HLA-B27-negative acute anterior uveitis. Am J Ophthalmol 120:351–361, 1995 50. Brewerton DA, Hart FD, Nicholls A et al: Ankylosing spondylitis and HLA-27. Lancet 1:904–907, 1973 51. Lee D, Barker S, Su W et al: The clinical diagnosis of Reiter's syndrome. Ophthalmology 93:350, 1986 52. Brewer EJ Jr, Bass J, Baum J et al: Current proposed revision of JRA Criteria. JRA Criteria Subcommittee of
the Diagnostic and Therapeutic Criteria Committee of the American Rheumatism
Section of The Arthritis Foundation. Arthritis Rheum 20:195–199, 1977 53. Cassidy JT, Levinson JE, Bass JC et al: A study of classification criteria for a diagnosis of juvenile rheumatoid
arthritis. Arthritis Rheum 29:274–281, 1986 54. Key SNd, Kimura SJ: Iridocyclitis associated with juvenile rheumatoid arthritis. Am J Ophthalmol 80:425–429, 1975 55. Kanski JJ: Anterior uveitis in juvenile rheumatoid arthritis. Arch Ophthalmol 95:1794–1797, 1977 56. Chylack LT Jr, Bienfang DC, Bellows AR, Stillman JS: Ocular manifestations of juvenile rheumatoid arthritis. Am J Ophthalmol 79:1026–1033, 1975 57. Dana MR, Merayo-Lloves J, Schaumberg DA et al: Visual outcomes prognosticators in juvenile rheumatoid arthritis-associated
uveitis. Ophthalmology 104:236–244, 1997 58. Rosenberg AM, Oen KG: The relationship between ocular and articular disease activity in children
with juvenile rheumatoid arthritis and associated uveitis. Arthritis Rheum 29:797–800, 1986 59. Kanski J: Juvenile arthritis and uveitis. Surv Ophthalmol 34:253, 1990 60. Foster CS, Barrett F: Cataract development and cataract surgery in patients with juvenile rheumatoid
arthritis-associated iridocyclitis. Ophthalmology 100:809–817, 1993 61. Ceisler EJ, Foster CS: Juvenile rheumatoid arthritis and uveitis: minimizing the blinding complications. Int Ophthalmol Clin 36:91–107, 1996 62. Olson NY, Lindsley CB, Godfrey WA: Nonsteroidal anti-inflammatory drug therapy in chronic childhood iridocyclitis. Am J Dis Child 142:1289–1292, 1988 63. Shetty A, Zganjar B, et al: Low-dose methotrexate in the treatment of severe juvenile rheumatoid arthritis
and sarcoid iritis. J Pediatr Ophthalmol Strabismus 36:125–128, 1999 64. Nguyen QD, Foster CS: Saving the vision of children with juvenile rheumatoid arthritis-associated
uveitis. JAMA 280:1133–1134, 1998 65. Giannini EH, Brewer EJ, Kuzmina N et al: Methotrexate in resistant juvenile rheumatoid arthritis: Results of the
U.S.A.-U.S.S.R. double-blind, placebo-controlled trial. The Pediatric
Rheumatology Collaborative Study Group and The Cooperative Children's
Study Group. N Engl J Med 326:1043–1049, 1992 66. Pavan-Langston D, Nelson DJ: Intraocular penetration of trifluridine. Am J Ophthalmol 87:814–818, 1979 67. Colin J, Malet F, Chastel C: Acyclovir in herpetic anterior uveitis. Ann Ophthalmol 23:28–30, 1991 68. Reijo A, Antti V, Jukka M: Endothelial cell loss in herpes zoster keratouveitis. Br J Ophthalmol 67:751–754, 1983 69. Cobo LM, Foulks GN, Liesegang T et al: Oral acyclovir in the therapy of acute herpes zoster ophthalmicus: An interim
report. Ophthalmology 92:1574–1583, 1985 70. Chern KC, Margolis TP: Varicella zoster virus ocular disease. Int Ophthalmol Clin 38:149–160, 1998 71. McGill J, Chapman C: A comparison of topical acyclovir with steroids in the treatment of herpes
zoster keratouveitis. Br J Ophthalmol 67:746–750, 1983 72. Givens KT, Lee DA, Jones T, Ilstrup DM: Congenital rubella syndrome: Ophthalmic manifestations and associated systemic
disorders. Br J Ophthalmol 77:358–363, 1993 73. Wolff SM: The ocular manifestations of congenital rubella. Trans Am Ophthalmol Soc 70:577–614, 1972 74. Boger WPd: Late ocular complications in congenital rubella syndrome. Ophthalmology 87:1244–1252, 1980 75. Brooks A, Gillies W: Glaucoma associated with congenital hypoplasia of the iris stroma in rubella. Glaucoma 11:36–41, 1989 76. Margo CE, Hamed LM: Ocular syphilis. Surv Ophthalmol 37:203–220, 1992 77. Tsukahara S: Secondary glaucoma due to inactive congenital syphilitic interstitial keratitis. Ophthalmologica 174:188, 1977 78. Smith JL: Testing for congenital syphilis in interstitial keratitis. Am J Ophthalmol 72:816–820, 1971 79. Grant W: Late glaucoma after interstitial keratitis. Am J Ophthalmol 79:87, 1975 80. Sugar H: Late glaucoma associated with inactive syphilitic interstitial keratitis. Am J Ophthalmol 53:602, 1962 81. Jabs DA, Johns CJ: Ocular involvement in chronic sarcoidosis. Am J Ophthalmol 102:297–301, 1986 82. Obenauf CD, Shaw HE, Sydnor CF, Klintworth GK: Sarcoidosis and its ophthalmic manifestations. Am J Ophthalmol 86:648–655, 1978 83. Iwata K, Nanba K, Sobue K, Abe H: Ocular sarcoidosis: Evaluation of intraocular findings. Ann NY Acad Sci 278:445–454, 1976 84. Dana MR, Merayo-Lloves J, Schaumberg DA, Foster CS: Prognosticators for visual outcome in sarcoid uveitis. Ophthalmology 103:1846–1853, 1996 85. Forster DJ, Rao NA, Hill RA et al: Incidence and management of glaucoma in Vogt-Koyanagi-Harada syndrome. Ophthalmology 100:613–618, 1993 86. Ohno S, Char DH, Kimura SJ, O'Connor GR: Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 83:735–740, 1977 87. Rathinam SR, Namperumalsamy P, Nozik RA, Cunningham ET Jr: Angle closure glaucoma as a presenting sign of Vogt-Koyanagi-Harada syndrome [letter]. Br J Ophthalmol 81:608–609, 1997 88. Eibschitz-Tsimhoni M, Gelfand YA, Mezer E, Miller B: Bilateral angle closure
glaucoma: An unusual presentation of Vogt- Koyanagi-Harada syndrome. Br
J Ophthalmol 81:705–706, 1997 (Letter) 89. Kimura R, Sakai M, Okabe H: Transient shallow anterior chamber as initial symptom in Harada's
syndrome. Arch Ophthalmol 99:1604–1606, 1981 90. Colvard DM, Robertson DM, O'Duffy JD: The ocular manifestations of Behçet's disease. Arch Ophthalmol 95:1813–1817, 1977 91. Benezra D, Cohen E: Treatment and visual prognosis in Behçet's disease. Br J Ophthalmol 70:589–592, 1986 92. James DG, Spiteri MA: Behçet's disease. Ophthalmology 89:1279–1284, 1982 93. Smith RE, Godfrey WA, Kimura SJ: Complications of chronic cyclitis. Am J Ophthalmol 82:277–282, 1976 94. Pederson JE, Kenyon KR, Green WR, Maumenee AE: Pathology of pars planitis. Am J Ophthalmol 86:762–774, 1978 95. Makley TA Jr, Azar A: Sympathetic ophthalmia. A long-term follow-up. Arch Ophthalmol 96:257–262, 1978 96. Harbin TS Jr, Pollack IP: Glaucoma in episcleritis. Arch Ophthalmol 93:948–950, 1975 97. Watson PG, McKay DA, Clemett RS, Wilkinson P: Treatment of episcleritis: A double-blind trial comparing betamethasone 0.1 percent
and oxyphenbutazone 10 percent placebo eye ointments. Br J Ophthalmol 57:866–870, 1973 98. Chandler P, Grant W: Inflammatory precipitates of the trabecular meshwork. In
Chandler PA, Grant WM (eds): Lectures on Glaucoma. Philadelphia: Lea & Febiger, 1965:257 99. Roth M, Simmons RJ: Glaucoma associated with precipitates on the trabecular meshwork. Ophthalmology 86:1613–1619, 1979 100. Levatin P: Glaucomatocyclitic crisis occurring in both eyes. Am J Ophthalmol 41:1056, 1956 101. Kass MA, Becker B, Kolker AE: Glaucomatocyclitic crisis and primary open-angle glaucoma. Am J Ophthalmol 75:668–673, 1973 102. Raitta C, Vannas A: Glaucomatocyclitic crisis. Arch Ophthalmol 95:608–612, 1977 103. Burnstein Y, Shelton K, Higginbotham EJ: Glaucomatocyclitic crisis in a child. Am J Ophthalmol 126:136–137, 1998 104. Teekhasaenee C, Ritch R: Glaucomatocyclitic crisis in a child [letter; comment]. Am J Ophthalmol 127:626–627, 1999 105. Posner A, Schlossman A: Further observations on the syndrome of glaucomatocyclitic crises. Trans Am Acad Ophthalmol Otolaryngol 57:531, 1953 106. Hart CT, Weatherill JR: Gonioscopy and tonography in glaucomatocyclitic crises. Br J Ophthalmol 52:682–687, 1968 107. Theodore F: Observations on glaucomatocyclitic crises. Br J Ophthalmol 36:207, 1952 108. Grant M: Clinical measurements of aqueous outflow. Arch Ophthalmol 46:113, 1951 109. Nagataki S, Mishima S: Aqueous humor dynamics in glaucomato-cyclitic crisis. Invest Ophthalmol 15:365–370, 1976 110. Spivey B, Armaly M: Tonographic findings in glaucomatocyclitic crises. Am J Ophthalmol 55:47, 1963 111. Eakins KE: Increased IOP produced by prostaglandins E1 and E2 in the cat eye. Exp Eye Res 10:87–92, 1970 112. Harstad HK, Ringvold A: Glaucomatocyclitic crises (Posner-Schlossman syndrome): A case report. Acta Ophthalmol (Copenh) 64:146–151, 1986 113. Bloch-Michel E, Dussaix E, Cerqueti P, Patarin D: Possible role of cytomegalovirus infection in the etiology of the Posner-Schlossmann
syndrome. Int Ophthalmol 11:95–96, 1987 114. Yamamoto S, Pavan-Langston D, Tada R et al: Possible role of herpes simplex virus in the origin of Posner-Schlossman
syndrome. Am J Ophthalmol 119:796–798, 1995 115. Knox DL: Glaucomatocyclitic crises and systemic disease: Peptic ulcer, other gastrointestinal
disorders, allergy and stress. Trans Am Ophthalmol Soc 86:473–495, 1988 116. Hung PT, Chang JM: Treatment of glaucomatocyclitic crises. Am J Ophthalmol 77:169–172, 1974 117. Fuchs E: Ueber Komplikationen der Heterochromie. Z Au-genheilkd 15:191, 1906 118. Jones N: Glaucoma in Fuchs' heterochromic uveitis: Aetiology, management and outcome. Eye 5:662–667, 1991 119. La Hey E, de Vries J, et al: Treatment and prognosis of secondary glaucoma in Fuchs' heterochromic iridocyclitis. Am J Ophthalmol 116:327–340, 1993 120. Tabbut BR, Tessler HH, Williams D: Fuchs' heterochromic iridocyclitis in blacks. Arch Ophthalmol 106:1688–1690, 1988 121. Franceschetti A: Heterochromic cyclitis. Am J Ophthalmol 39:50, 1955 122. O'Connor GR: Doyne Lecture. Heterochromic iridocyclitis. Trans Ophthalmol Soc UK 104:219–231, 1985 123. Perry HD, Yanoff M, Scheie HG: Rubeosis in Fuchs heterochromic iridocyclitis. Arch Ophthalmol 93:337–39, 1975 124. Kimura S, Hogan M: Fuchs' syndrome of heterochromic cyclitis. Arch Ophthalmol 54:179, 1955 125. Jones N: Fuchs' heterochromic uveitis: A reappraisal of the clinical spectrum. Eye 5:649–661, 1991 126. Makley T: Heterochromic cyclitis in identical twins. Am J Ophthalmol 41:768, 1956 127. Jones N, Read A: Is there a genetic basis for Fuchs' heterochromic uveitis? Discordance
in monozygotic twins. Br J Ophthalmol 76:22, 1992 128. Kimura SJ: Fuchs' syndrome of heterochromic cyclitis in brown-eyed patients. Trans Am Ophthalmol Soc 76:76–89, 1978 129. Rothova A, La Hey E, Baarsma GS, Breebaart AC: Iris nodules in Fuchs' heterochromic uveitis. Am J Ophthalmol 118:338–342, 1994 130. Goldberg MF, Erozan YS, Duke JR, Frost JK: Cytopathologic and histopathologic aspects of Fuchs' heterochromic iridocyclitis. Arch Ophthalmol 74:604–609, 1965 131. McCartney AC, Bull TB, Spalton DJ: Fuchs' heterochromic cyclitis: An electron microscopy study. Trans Ophthalmol Soc UK 105:324–329, 1986 132. Soheilian M, Karimian F, et al: Surgical management of cataract and posterior chamber intraocular lens
implantation in Fuchs' heterochromic iridocyclitis. Int Ophthalmol 21:137, 1997 133. Lerman S, Levy C: Heterochromic cyclitis. Am J Ophthalmol 57:479, 1964 134. Ward D, Hart C: Complicated cataract extraction in Fuchs' heterochromic uveitis. Br J Ophthalmol 51:530, 1967 135. Feldman ST, Deutsch TA: Hyphema following Honan balloon use in Fuchs' heterochromic
iridocyclitis. Arch Ophthalmol 104:967, 1986 (Letter) 136. Smith RE, O'Connor GR: Cataract extraction in Fuchs syndrome. Arch Ophthalmol 91:39–41, 1974 137. Amsler M, Verrey F: Heterochromie de Fuchs et fragilite vasculaire. Ophthalmologica 111:177, 1946 138. Toledo de Abreu M, Belfort R Jr, Hirata PS: Fuchs' heterochromic cyclitis and ocular toxoplasmosis. Am J Ophthalmol 93:739–744, 1982 139. Schwab IR: The epidemiologic association of Fuchs' heterochromic iridocyclitis and
ocular toxoplasmosis. Am J Ophthalmol 111:356–362, 1991 140. Arffa RC, Schlaegel TF Jr: Chorioretinal scars in Fuchs' heterochromic iridocyclitis. Arch Ophthalmol 102:1153–1155, 1984 141. La Hey E, Rothova A, Baarsma GS et al: Fuchs' hetero-chromic iridocyclitis is not associated with ocular toxoplasmosis. Arch Ophthalmol 110:806–811, 1992 142. Daus W, Schmidbauer J, et al: Results of extracapsular cataract extraction with intraocular lens implantation
in eyes with uveitis and Fuchs' heterochromic iridocyclitis. German J Ophthalmol 1:399, 1992 143. Baarsma GS, de Vries J, Hammudoglu CD: Extracapsular cataract extraction with posterior chamber lens implantation
in Fuch's heterochromic cyclitis. Br J Ophthalmol 75:306–308, 1991 144. Avramides S, Sakkias G, Traianidis P: Cataract surgery in Fuchs' heterochromic iridocyclitis. Eur J Ophthalmol 7:149, 1997 145. O'Neill D, Murray PI, Patel BC, Hamilton AM: Extracapsular cataract surgery with and without intraocular lens implantation
in Fuchs heterochromic cyclitis. Ophthalmology 102:1362–1368, 1995 146. Jones NP: Cataract surgery in Fuchs' heterochromic uveitis: Past, present, future. J Cataract Refract Surg 22:261–268, 1996 147. Saari M, Vuorre I, Nieminen H: Fuchs's heterochromic cyclitis: A simultaneous bilateral fluorescein
angiographic study of the iris. Br J Ophthalmol 62:715–721, 1978 148. Perkins E: Heterochromic uveitis. Trans Ophthalmol Soc UK 81:53, 1962 149. Jammes JL, Nigam MP: Pupillary autonomic functions in heterochromia iridis. Arch Ophthalmol 89:291–292, 1973 150. Van der Gaag R, Broersma L, Rothova A et al: Immunity to a corneal antigen in Fuchs' heterochromic cyclitis patients. Invest Ophthalmol Vis Sci 30:443–448, 1989 151. La Hey E, Baarsma G, Rothova A, et al: High incidence of corneal epithelium antibodies in Fuch's heterochromic
cyclitis. Br J Ophthalmol 72:921–925, 1988 152. Spinelli HM, Krohn DL: Inhibition of prostaglandin-induced iritis. Topical indoxole vs. indomethacin
therapy. Arch Ophthalmol 98:1106–1109, 1980 153. Marsettio M, Siverio CE, Oh JO: Effect of aspirin and dexamethasone on IOP in primary uveitis produced
by Herpes simplex virus. Am J Ophthalmol 81:636–641, 1976 154. Wong V, Hersh E: Methotrexate in the therapy of cyclitis. Trans Am Acad Ophthalmol Otolaryngol 69:279, 1965 155. Andrasch RH, Pirofsky B, Burns RP: Immunosuppressive therapy for severe chronic uveitis. Arch Ophthalmol 96:247–251, 1978 156. Akingbehin T, Villada J: Metipranolol-associated granulomatous anterior uveitis. Br J Ophthalmol 75:519–523, 1991 157. Patel NP, Patel KH, Moster MR, Spaeth GL: Metipranolol-associated nongranulomatous anterior uveitis. Am J Ophthalmol 123:843–844, 1997 158. Melles RB, Wong IG: Metipranolol-associated granulomatous iritis. Am J Ophthalmol 118:712–715, 1994 159. Galin M: Hypotensive effect of urea in inflamed and uninflamed eye. Arch Ophthalmol 68:633, 1962 160. Fechtner RD, Khouri AS, Zimmerman TJ et al: Anterior uveitis associated with latanoprost. Am J Ophthalmol 126:37–41, 1998 161. Warwar RE, Bullock JD, Ballal D: Cystoid macular edema and anterior uveitis associated with latanoprost
use: Experience and incidence in a retrospective review of 94 patients. Ophthalmology 105:263–268, 1998 162. Robin AL, Pollack IP: A comparison of neodymium-YAG and argon laser iridotomies. Ophthalmology 91:1011–1016, 1984 163. Lieberman MF, Hoskins HD Jr, Hetherington J Jr: Laser trabeculoplasty and the glaucomas. Ophthalmology 90:790–795, 1983 164. Campbell DG, Vela A: Modern goniosynechialysis for the treatment of synechial angle-closure
glaucoma. Ophthalmology 91:1052–1060, 1984 165. Kitazawa Y, Kawase K, Matsushita H, Minobe M: Trabeculectomy with mitomycin: a comparative study with fluorouracil. Arch Ophthalmol 109:1693–1698, 1991 166. Patitsas CJ, Rockwood EJ, Meisler DM, Lowder CY: Glaucoma filtering surgery with postoperative 5-fluorouracil in patients
with intraocular inflammatory disease. Ophthalmology 99:594–599, 1992 167. Jampel HD, Jabs DA, Quigley HA: Trabeculectomy with 5-fluorouracil for adult inflammatory glaucoma. Am J Ophthalmol 109:168–173, 1990 168. Towler H, Bates A, et al: Primary trabeculectomy with 5-fluorouracil for glaucoma secondary to uveitis. Ocular Immunol Inflamm 3:163, 1995 169. Wright MM, McGehee RF, Pederson JE: Intraoperative mitomycin-C for glaucoma associated with ocular inflammation. Ophthalmic Surg Lasers 28:370–376, 1997 170. Prata JA Jr, Neves RA, Minckler DS et al: Trabeculectomy with mitomycin C in glaucoma associated with uveitis. Ophthalmic Surg 25:616–620, 1994 171. Cheung JC, Wright MM, Murali S, Pederson JE: Intermediate-term outcome of variable dose mitomycin C filtering surgery. Ophthalmology 104:143–149, 1997 172. Katz GJ, Higginbotham EJ, Lichter PR et al: Mitomycin C versus 5-fluorouracil in high-risk glaucoma filtering surgery. Extended
follow-up. Ophthalmology 102:1263–1269, 1995 173. Hill RA, Nguyen QH, Baerveldt G et al: Trabeculectomy and Molteno implantation for glaucomas associated with uveitis. Ophthalmology 100:903–908, 1993 174. Gil-Carrasco F, Salinas-Van Orman E, Recillas-Gispert C et al: Ahmed valve implant for uncontrolled uveitic glaucoma. Ocul Immunol Inflamm 6:27–37, 1998 175. Da Mata A, Burk SE, Netland PA et al: Management of uveitic glaucoma with Ahmed glaucoma valve implantation. Ophthalmology 106:2168–2172, 1999 176. Siegner SW, Netland PA, Urban RC Jr et al: Clinical experience with the Baerveldt glaucoma drainage implant. Ophthalmology 102:1298–1307, 1995 177. Kanski JJ, McAllister JA: Trabeculodialysis for inflammatory glaucoma in children and young adults. Ophthalmology 92:927–930, 1985 178. McLean J: Clinical and experimental observation on the use of ACTH and cortisone
in ocular inflammatory disease: Discussion of a paper by Woods, AC. Trans Am Ophthalmol Soc 48:293, 1950 179. Francois J: Cortisone et tension oculaire. Ann Ocul 187:805, 1954 180. BenEzra D, Wysenbeek Y, Cohen E: Increased IOP during treatment for chronic uveitis. Graefes Arch Clin Exp Ophthalmol 235:200, 1997 181. Armaly M: Statistical attributes of the steroid hypertensive response in the clinically
normal eye. Invest Ophthalmol 4:187, 1965 182. Becker B: IOP response to topical corticosteroids. Invest Ophthalmol 4:198–205, 1965 183. Armaly MF: Effects of corticosterids on IOP and fluid dynamics. II. The effects of
dexamethasone in the glaucomatous eye. Arch Ophthalmol 70:492–499, 1963 184. Podos SM, Becker B, Morton WR: High myopia and primary open-angle glaucoma. Am J Ophthalmol 62:1038–1043, 1966 185. Becker B: Diabetes mellitus and primary open-angle glaucoma. Am J Ophthalmol 71:1–16, 1971 186. Gaston H, Absolon MJ, Thurtle OA, Sattar MA: Steroid responsiveness in connective tissue diseases. Br J Ophthalmol 67:487–490, 1983 187. Ohji M, Kinoshita S, Ohmi E, Kuwayama Y: Marked IOP response to instillation of corticosteroids in children. Am J Ophthalmol 112:450–454, 1991 188. Biedner BZ, David R, Grudsky A, Sachs U: IOP response to corticosteroids in children. Br J Ophthalmol 64:430–431, 1980 189. Becker B, Mills DW: Corticosteroids and IOP. Arch Ophthalmol 70:500–507, 1963 190. Francois J: Corticosteroid glaucoma. Ophthalmologica 188:76–81, 1984 191. Weinreb RN, Polansky JR, Kramer SG, Baxter JD: Acute effects of dexamethasone on IOP in glaucoma. Invest Ophthalmol Vis Sci 26:170–175, 1985 192. Weinreb RN, Bloom E, Baxter JD et al: Detection of glucocorticoid receptors in cultured human trabecular cells. Invest Ophthalmol Vis Sci 21:403–407, 1981 193. Francois J: The importance of the mucopolysaccharides in IOP regulation. Invest Ophthalmol 14:173–176, 1975 194. Zugerman C, Saunders D, Levit F: Glaucoma from topically applied steroids. Arch Dermatol 112:1326, 1976 195. Cubey RB: Glaucoma following the application of corticosteroid to the skin of the
eyelids. Br J Dermatol 95:207–208, 1976 196. Spaeth GL, Rodrigues MM, Weinreb S: Steroid-induced glaucoma: A. Persistent elevation of IOP. B. Histopathological
aspects. Trans Am Ophthalmol Soc 75:353–381, 1977 197. Stern J: Acute glaucoma during cortisone therapy. Am J Ophthalmol 36:389, 1953 198. Covell L: Glaucoma induced by systemic steroid therapy. Am J Ophthalmol 45:108, 1958 199. Godel V, Feiler-Ofry V, Stein R: Systemic steroids and ocular fluid dynamics. I. Analysis of the sample
as a whole. Influence of dosage and duration of therapy. Acta Ophthalmol 50:655–663, 1972 200. Haas JS, Nootens RH: Glaucoma secondary to benign adrenal adenoma. Am J Ophthalmol 78:497–500, 1974 201. Herschler J: Increased IOP induced by repository corticosteroids. Am J Ophthalmol 82:90–93, 1976 202. Helm CJ, Holland GN: The effects of posterior subtenon injection of triamcinolone acetonide
in patients with intermediate uveitis. Am J Ophthalmol 120:55–64, 1995 203. Mueller AJ, Jian G, Banker AS et al: The effect of deep posterior subtenon injection of corticosteroids on IOP. Am J Ophthalmol 125:158–163, 1998 204. Akduman L, Kolker AE, Black DL et al: Treatment of persistent glaucoma secondary to periocular corticosteroids. Am J Ophthalmol 122:275–277, 1996 205. Dreyer EB: Inhaled steroid use and glaucoma [letter].N Engl J Med 329:1822, 1993 206. Garbe E, LeLorier J, Boivin JF, Suissa S: Inhaled and nasal glucocorticoids and the risks of ocular hypertension
or open-angle glaucoma. JAMA 277:722–727, 1997 207. Mitchell P, Cumming RG, Mackey DA: Inhaled corticosteroids, family history and risk of glaucoma. Ophthalmology 106:2301–2306, 1999 208. Opatowsky I, Feldman RM, Gross R, Feldman ST: IOP elevation associated with inhalation and nasal corticosteroids. Ophthalmology 102:177–179, 1995 209. Podos SM, Krupin T, Asseff C, Becker B: Topically administered corticosteoid preparations. Comparison of IOP effects. Arch Ophthalmol 86:251–254, 1971 210. Fairbairn WD, Thorson JC: Fluorometholone. Anti-inflammatory and IOP effects. Arch Ophthalmol 86:138–141, 1971 211. Morrison E, Archer DB: Effect of fluorometholone (FML) on the IOP of corticosteroid responders. Br J Ophthalmol 68:581–584, 1984 212. Leibowitz HM, Ryan WJ Jr, Kupferman A: Comparative anti-inflammatory efficacy of topical corticosteroids with
low glaucoma-inducing potential. Arch Ophthalmol 110:118–120, 1992 213. Drance SM, Scott S: A comparison of action of dexamethasone and medrysone on human IOP. Can J Ophthalmol 3:159–161, 1968 214. Bedrossian RH, Eriksen SP: The treatment of ocular inflammation with medrysone. Arch Ophthalmol 81:184–191, 1969 215. Foster CS, Alter G, DeBarge LR et al: Efficacy and safety of rimexolone 1% ophthalmic suspension vs. 1% prednisolone
acetate in the treatment of uveitis. Am J Ophthalmol 122:171–182, 1996 216. Leibowitz HM, Bartlett JD, Rich R et al: IOP-raising potential of 1.0% rimexolone in patients responding to corticosteroids. Arch Ophthalmol 114:933–937, 1996 217. Novack GD, Howes J, Crockett RS, Sherwood MB: Change in IOP during long-term use of loteprednol etabonate.J Glaucoma 7:266–269, 1998 218. Loteprednol Etabonate US Uveitis Study Group: Controlled evaluation of
loteprednol etabonate and prednisolone acetate in the treatment of acute
anterior uveitis. Am J Ophthalmol 127:537–544, 1999 219. Spiers F: A case of irreversible steroid-induced rise in IOP. Acta Ophthalmol 43:419, 1965 220. Gieser DK, Hodapp E, Goldberg I et al: Flurbiprofen and IOP. Ann Ophthalmol 13:831–833, 1981 |