MULTIFACTORIAL LINKAGE
Glaucoma occurs at a higher frequency in patients with certain retinal disorders than might be expected in an age-matched control group. The retinal disorder itself does not appear to have a direct role in the glaucoma, but the frequency of the association and the clinical relevance bear consideration.
Retinal Detachments
In the general population the incidence of primary open-angle glaucoma approaches 1%1; by comparison Becker reported 5.8% of patients with retinal detachments (31 of 530 patients) had unrecognized primary open-angle glaucoma in the contralateral eye, with the diagnosis based on disc analysis, visual fields, tonometry, and/or tonography.2 In a review of 817 patients undergoing primary retinal detachment repair, Phelps and Burton found glaucoma in 9.5% (78 of 817 patients); the glaucoma preceded the retinal detachment historically or by clinical evidence in 7.3% (60 of 817 patients) and followed the retinal detachment in 2.2% (18 of 817 patients).3 The reason for the association between retinal detachment and glaucoma was unclear and, surprisingly, the authors could find no definite risk factors in myopia, trauma, or the use of miotics. In aphakic eyes, however, the risk of glaucoma was three to four times more likely compared with the phakic group.
Shammas and colleagues reviewed glaucoma risk factors in 30 patients with primary, atraumatic retinal detachments. Following the chronic administration of topical dexamethasone drops to the contralateral, nondetached eye, they found that 20% (6 of 30 patients) had an intraocular pressure rise of 16 mm Hg or higher.4 This was a significantly higher proportion than the expected 5% to 6% high-pressure corticosteroid responders reported previously in a general population group supporting a strong predisposition in patients with retinal detachment.5,6
A possible association between the use of miotics and acute retinal detachments has been suggested in numerous reports.7–9 These agents were of more clinical significance in the past, when they were a mainstay of glaucoma treatment, and the authors now limit their use to pseudophakic patients in which other agents have failed. Regardless, the association with retinal detachment should be kept in mind, particularly in susceptible patients (ex: high myopia).
One study of patients with pigment dispersion syndrome (PDS) showed an increased prevalence of lattice changes and retinal detachment as compared to non-PDS patients having a similar distribution of myopic eyes.10 For this reason, a good peripheral examination in such patients is warranted, and recently symptomatic patients should be treated. This association may be genetically determined, either by a single gene or by two genes at closely linked loci. Unique anatomical variations at both the peripheral iris and vitreous base may explain this increased risk.10
Retinitis Pigmentosa
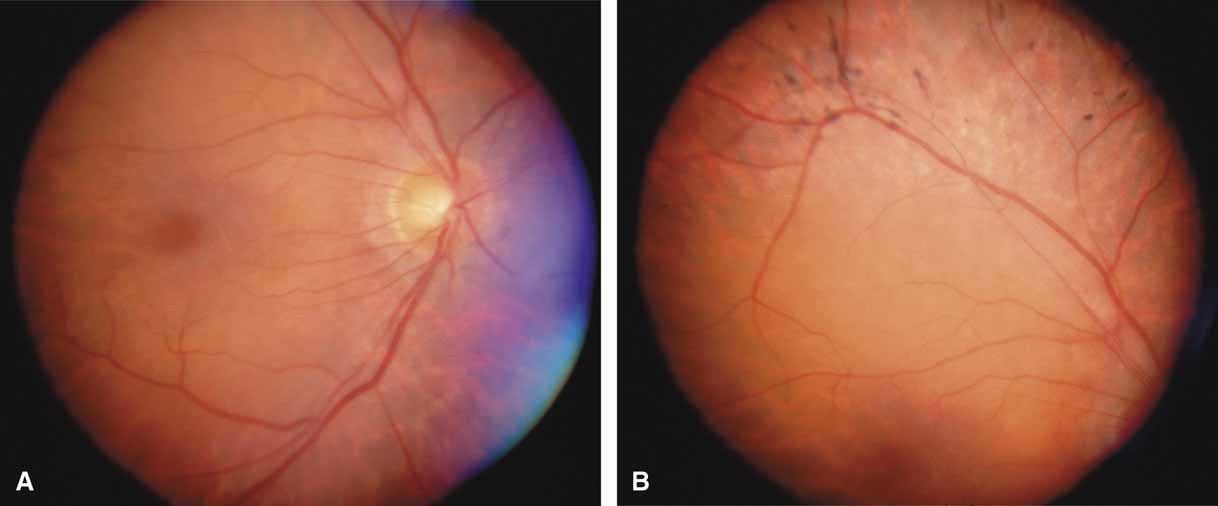
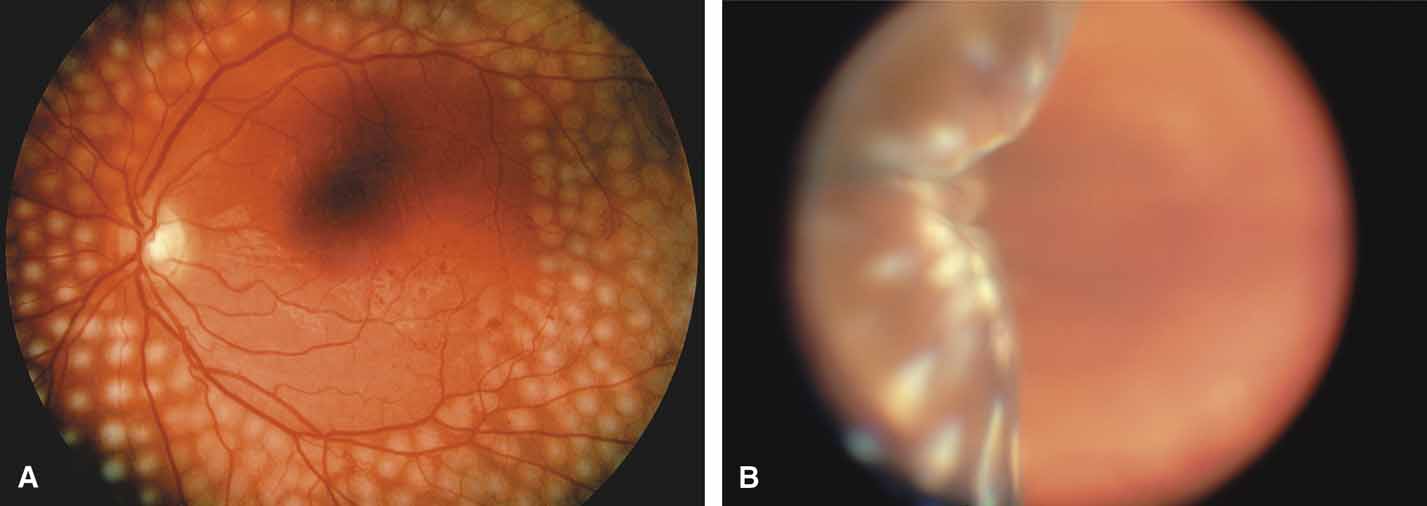
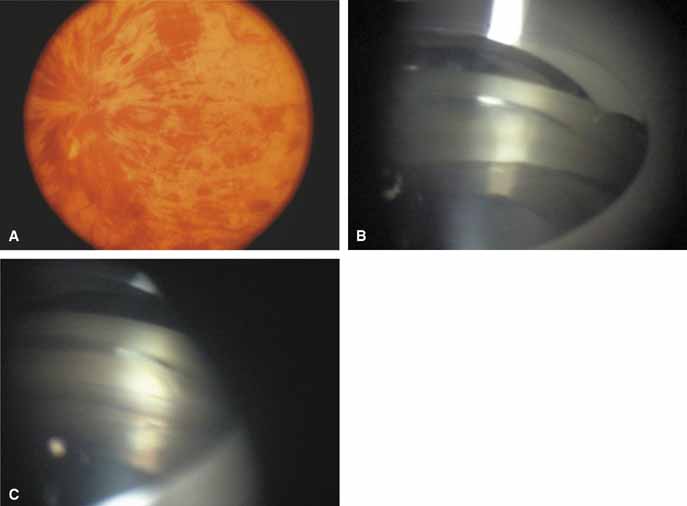
The possible association between retinitis pigmentosa and glaucoma dates back to 1862.11 Over the past century the reported glaucoma incidence has varied between 2.98% to 10% of all cases of retinitis pigmentosa.12,13 Of concern in all previous reports is the accuracy of the diagnosis of retinitis pigmentosa itself, a hereditary disease with diverse manifestations. Retinitis pigmentosa-like syndromes may mimic glaucomatous field loss, and the pattern of advancement of visual field loss in true retinitis pigmentosa may parallel that of typical open-angle glaucoma. Patients with undiagnosed retinitis pigmentosa may have subtle fundus findings, especially those with the sine pigmento form (Fig. 1). A clue to diagnosis is the disproportionate peripheral field loss in comparison to the degree of optic nerve cupping and rim pallor. Patients with atypical field loss and minimal optic nerve cupping should have an electroretinogram to exclude unsuspected retinitis pigmentosa.
Stickler's Syndrome
Glaucoma has been reported in Stickler's vitreoretinopathy, an autosomal dominant disorder that demonstrates multiple systemic abnormalities in addition to the ocular abnormalities emphasized in seminal reports by Wagner14 and Janssen.15 Ophthalmic manifestations include an optically clear vitreous with prominent peripheral vitreoretinal membranes that have been associated with retinal detachment. High myopia, nuclear sclerotic cataracts, and glaucoma complete the eye findings. Systemic findings include flattened facies, high-arched palate, hearing loss, scapular abnormalities, and knee pain due to spondyloepiphyseal dysplasia. The glaucoma has been described as an open-angle type with both congenital and later-onset varieties. Phelps16 has reported normal angles on gonioscopy and a good response to medication. He recommended avoiding miotics in light of the peripheral retinal abnormalities, high myopia, and potentially higher risk of retinal detachment. Neovascular glaucoma has been reported, but occurs most often following multiple surgeries.17
OBSTRUCTIVE/PARTICULATE MATTER
Particulate matter of varying sizes can cause a mechanical obstruction to outflow or in other ways damage the trabecular meshwork of an otherwise normal open-angle. The degree and duration of the pressure elevation, the underlying health of the optic nerve, and systemic factors determine the outcome and the ultimate effect on vision.
Uveitis
Disorders in this category affect the eye and adnexa in three general locations that frequently overlap: (1) anterior segment, (2) posterior segment, and (3) orbit (Table 2).18 Involvement of the anterior segment may produce a high, low, or normal intraocular pressure, depending on the balance between inflow and outflow.
TABLE 2. Mechanisms of Glaucoma in Uveitis
| Increased viscosity of aqueous humor |
| Obstruction of the trabecular meshwork |
| Swelling and dysfunction of the trabecular meshwork |
| Liberation of active substances such as prostaglandins and substance P |
| Scarring of the outflow channels |
| Development of a cuticular membrane over the angle |
| Neovascularization |
| Elevation of episcleral venous pressure |
| Forward movement of the iris lens diaphragm |
| Pupillary block |
| Formation of peripheral anterior synechiae |
(Modified from Hoskins HD Jr, Kass M: Becker-Schaffer's diagnosis and therapy of the glaucomas. 6th ed. St Louis: CV Mosby, 1989)
Specific posterior segment entities known to cause glaucoma include sarcoidosis, toxoplasmosis, and syphilis. In a study of 202 patients with sarcoid affecting the eyes, 10.9% (22 of 202) were found to have glaucoma at some time during the clinical course of the illness.19 Lichter20 reported that syphilis was associated with glaucoma of three distinct types: (1) iritic, which demonstrated peripheral anterior synechiae, a residua of inflammation; (2) narrow angle; and (3) irregular angle-closure, in which intraepithelial cysts of the ciliary body and iris are seen on gonioscopy and account for the variable narrowing of the angle approach.
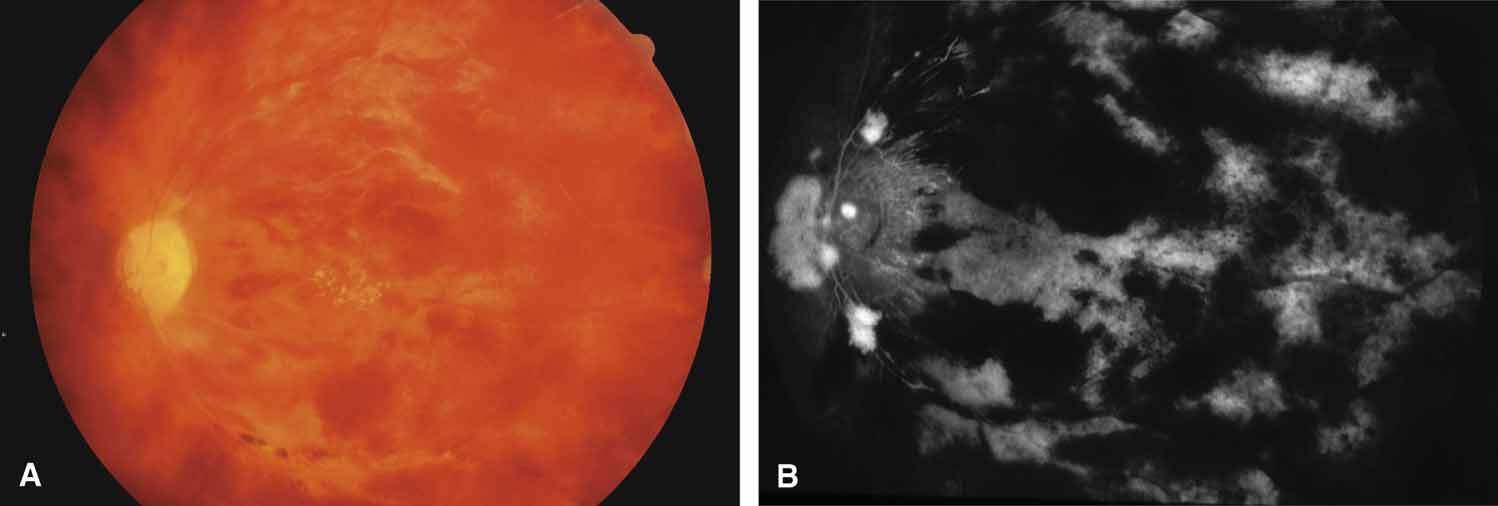
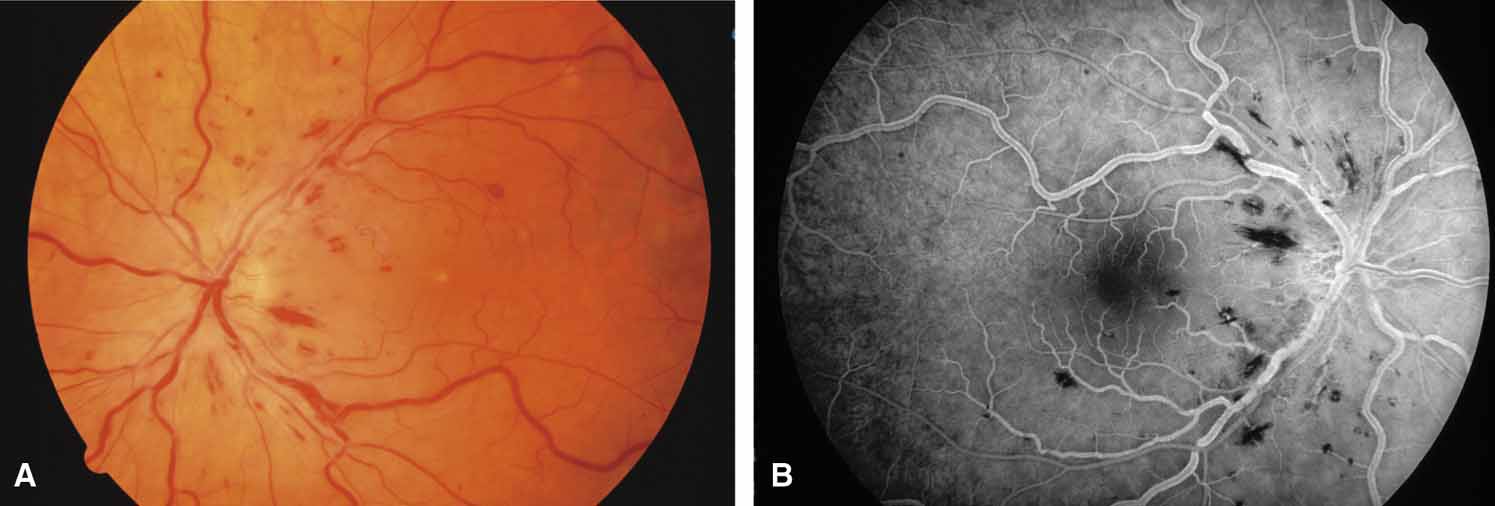
Active retinal involvement in acute toxoplasmosis may be associated with an acute elevation of intraocular pressure, sometimes producing corneal edema. When the cornea is cleared with topical glycerin, the angle is found to be open and a minimal anterior chamber reaction is present. The mechanism of the glaucoma is believed to be immune complex (antigen–antibody) deposition in the trabecular meshwork, rather than obstruction by cellular debris.21 The elevated pressure responds well to timolol or acetazolamide and does not require topical corticosteroids. Systemic treatment of the active retinitis should follow only if the optic nerve (positive Marcus Gunn), macular center, or peripapillary retina is involved. This unique entity will be missed if a fundus examination is not performed in all cases of hypertensive uveitis (Fig. 2).
Active anterior or posterior uveitis, although minimally active, may upset the inflow/outflow balance severely and produce pressure elevations out of proportion to the anterior chamber reaction. Careful gonioscopy may reveal a localized trabeculitis manifesting as an occasional keratic precipitate on the trabecular meshwork or more anteriorly on the corneal endothelium. This easily overlooked syndrome mimics a medically unresponsive open-angle glaucoma and is important to correctly diagnose because the pressure can be lowered with the application of topical corticosteroids.22 In corticosteroid-sensitive persons, topical and periocular corticosteroid-related pressure elevations may become intractable. Long-acting depot preparations may require surgical removal of the depot corticosteroid to ameliorate the pressure elevation.23
Treatment with corticosteroids may hasten the recovery from hypotony in dramatic fashion, producing an acute pressure elevation as the blood aqueous barrier recovers and inflow overwhelms outflow. For this reason, careful titration of the corticosteroid dosage and simultaneous observation of the effect on intraocular pressure is advised.
Red Blood Cells
Long-standing vitreous hemorrhage can produce an acute glaucoma of an open-angle type. Originally, the term hemolytic glaucoma was employed to describe these events because pathologic studies showed macrophages containing hemoglobin in the anterior chamber.24 The situation was thought to be analogous to phacolytic glaucoma (hypermature liquefied lens protein engulfed by macrophages). Later, Campbell and co-workers25,26 demonstrated that the major mechanism was trabecular meshwork obstruction by ghost cells, rather than red blood cells, hemoglobin, or macrophages. Red blood cells in the vitreous release their hemoglobin within a few days and become the less pliable membranous container called a ghost cell. For simplicity, we consider the conditions hemolytic glaucoma and ghost cell glaucoma to be interchangeable. Two other disorders, hemosiderotic and siderotic glaucoma, add confusion to the terminology. These entities are separate glaucomatous disorders due to iron-induced damage of an open trabecular meshwork. The source of the iron may be blood (hemosiderotic) or an occult iron-containing foreign body (siderotic).
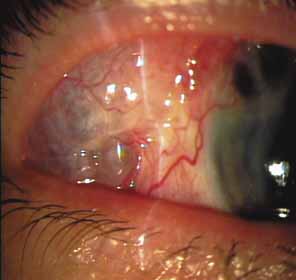
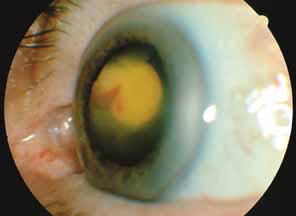
The majority of cases of ghost cell glaucoma occur following vitrectomy for diabetic retinopathy, vitreous hemorrhage due to trauma, or vitreous hemorrhage following anterior segment surgery (i.e., cataract extraction or corneal transplant). Although studies suggest that a disruption of the anterior hyaloid face may be a necessary precedent, ghost cell glaucoma probably can occur with an intact hyaloid if a sufficient quantity of vitreous blood is present.27 The clinical presentation includes acute pressure elevation, corneal edema, and lack of keratic precipitates. Minute tan cells (ghost cells) may layer out, producing a pseudohypopyon or, if admixed with blood, a “candy-striped” hypopyon. (Fig. 3) Intraocular pressure elevations after diabetic vitrectomy raise concerns regarding concomitant neovascular glaucoma. However, neovascular glaucoma rarely develops in the immediate postvitrectomy period, unless some anterior segment neovascularization was present preoperatively. Late-onset pressure elevations (> 6 to 8 weeks out) in a postvitrectomized diabetic eye usually signal neovascular glaucoma.
|
Blunt trauma that produces hyphema and vitreous hemorrhage may result in an early pressure elevation (hyphema-induced), which abates in 1 to 2 weeks. If the pressure becomes elevated in the absence of hyphema, the diagnosis of ghost cell glaucoma secondary to residual vitreous hemorrhage should be considered. One should look for fine cells that are smaller than those seen in uveitis and are more uniform and larger than pigment particles. Blunt trauma may also cause angle recession glaucoma, which is usually asymptomatic and can occur weeks, months, or even years later.
Intraocular hemorrhage after anterior segment surgery such as cataract extraction or corneal transplant may be confused with infectious endophthalmitis, which may also result in acute pressure elevations. In clinically equivocal situations, diagnostic vitreous and aqueous smears and cultures and antibiotic therapy may be necessary.
In the treatment of red blood cell–related glaucoma, maximally tolerated medical treatment (Beta Blockers, Alpha 2 Agonists, Acetazolamide) and patience will usually result in a spontaneous abatement of the intraocular pressure elevation. Some patients with severe, unremitting intraocular pressure elevation or those developing corneal bloodstaining will require an anterior chamber washout or vitrectomy. If debris (blood or ghost cells) is present in the anterior chamber, visualization will require anterior chamber lavage prior to vitrectomy. The decision for surgery should be guided by a prior knowledge of the health of the optic nerve, the clarity of the cornea, and the comfort of the patient. A history of sickle cell disorders requires a more aggressive approach to preserve optic nerve perfusion which is more easily compromised by elevated intraocular pressure and systemic use of acetazolamide (produces metabolic acidosis).28 Hemoglobin electrophoresis or a sickle cell preparation is recommended to diagnose the disorder in populations at risk for this hemoglobinopathy.
Tumors
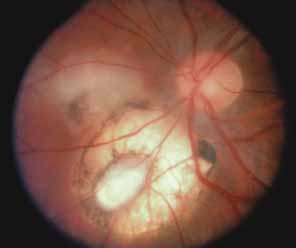
Both benign and malignant tumors can produce a secondary glaucoma. Shields and associates29 found 5% (126) of 2,704 eyes diagnosed with intraocular tumors suffered glaucoma. The glaucoma rate was only 2% in eyes with choroidal melanomas but rose to 17% in eyes with melanomas of the ciliary body.28 The mechanism of glaucoma in posterior segment melanomas was usually iris neovascularization (Fig. 4; Table 3).30 The incidence of glaucoma in tumor-containing eyes was found to rise when retinal detachment was present. In eyes presenting with reduced vision, glaucoma, opaque media, and an uncertain history of visual loss, it is prudent to exclude an unsuspected neoplasm by performing diagnostic B-scan ultrasonography.
TABLE 3. Glaucoma Mechanisms in Eyes Containing Uveal Malignant Melanoma
| Mechanism | Underlying Cause |
| Peripheral anterior synechiae and angle closure | Posterior synechiae, iris bombe and peripheral anterior synechiae |
| Rubeosis irides and peripheral anterior synechiae | |
| Diffuse iris nevus or melanoma and peripheral anterior synechiae | |
| Cellular obstruction of aqueous drainage area of an open angle | Seeding of neoplasm into anterior chamber angle |
| Ring melanoma with invasion of anterior chamber angle structures | |
| Melanin phagocytosis by macrophages with obstruction of anterior chamber angle (melanomalytic glaucoma) |
(Yanoff M: Glaucoma mechanisms in eyes containing uveal malignant melanoma. Am J Ophthalmol 70:898, 1970)
Melanomalytic glaucoma is the term applied to glaucoma due to macrophages laden with pigment released from necrosis of a ciliary body melanoma.31 However, because a similar picture may occur with a nonmalignant tumor (melanocytoma), the presence of glaucoma associated with a pigmented mass does not itself confirm the diagnosis of malignancy. Posterior segment metastases caused a pressure elevation due to angle closure–related choroidal mass effect or retinal detachment in only 1% of eyes.29
Leukemic involvement of the eye generally affects the choroid and retina, but iris infiltration, hypopyon, and glaucoma have been reported; irradiation is often successful in these cases.32
Schwartz Syndrome (Rod Outer Segment Obstruction)
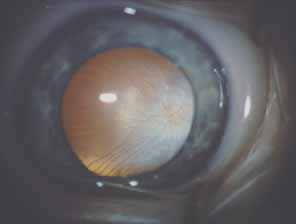
In 1973, Schwartz described 11 patients with a unilateral elevation of intraocular pressure, ranging from 29 to 55 mm Hg, who were found to have a rhegmatogenous retinal detachment that in some cases had been overlooked previously.33 Schwartz found the angles were open and normal except for one patient with an angle recession (5 of 11 patients had a history of trauma). The elevated pressures responded poorly to glaucoma medications given prior to the correct diagnosis, and successful retinal repair resulted in a normal intraocular pressure and outflow facility. More recent work34,35 suggests that trabecular obstruction by photoreceptor outer segments may play a role, although reduced aqueous outflow due to pigment dispersion and trabecular damage from previous trauma are all possible mechanisms of the glaucoma in this syndrome. This unique entity should be differentiated from coexistent primary open-angle glaucoma, which is usually bilateral, and from uveitic glaucoma, which generally responds to corticosteroids. It is important to exclude an occult retinal detachment in any medically unresponsive unilateral glaucoma especially prior to instituting miotics. Chronic miotic instillation may impair pupil size and compromise future retinal evaluation.
Silicone Oil/Gas
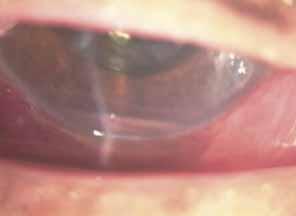
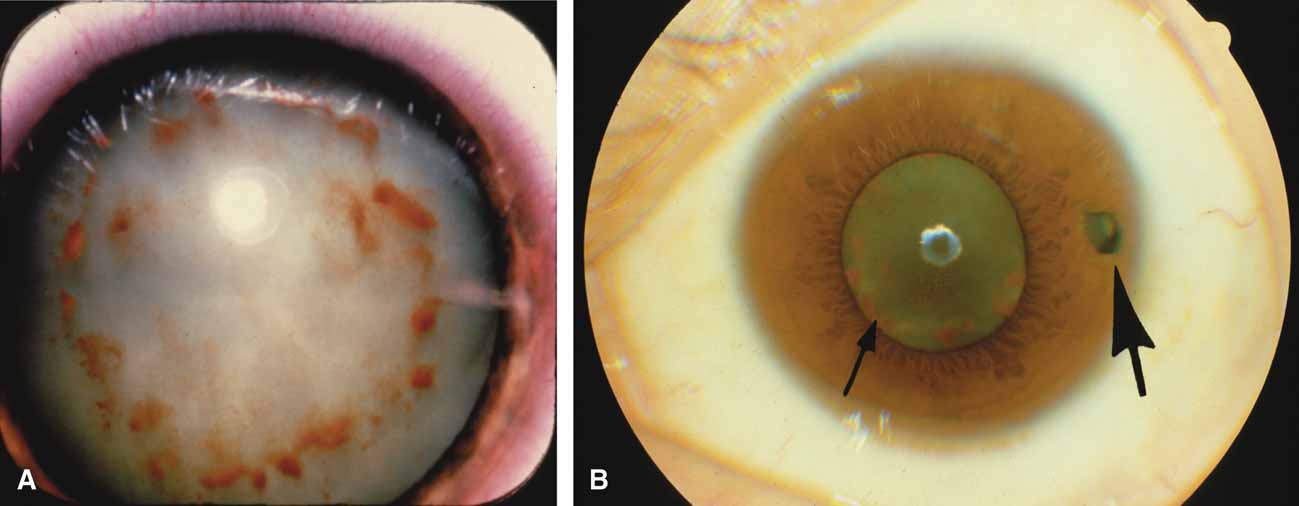
Over the past two decades silicone oil (dimethylpolysiloxane) has enjoyed a resurgence in the repair of complicated retinal detachments. In 1967, Watzke36 found no pressure elevation attributable to silicone but described droplets in the superior angle that result from silicone's lower specific gravity (buoyancy) compared with water. Silicone-induced glaucoma does occur, however, and one possible mechanism is an acute pupillary block due to the buoyancy of the silicone sometimes combined with overfilling the aphakic eye (Fig. 5). This can be averted by performing an inferior iridectomy and by paying careful attention to the silicone volume injected. A second mechanism involves chronic obstruction of an open angle by emulsified silicone microbubbles (more likely with low viscosity silicone, 1,000 centistokes, than high viscosity silicone, 12,500 centistokes) (Fig. 6), pigmented cells, and silicone-laden macrophages or silicone-induced fibrosis of the trabecular meshwork due to possible inherent fibrogenicity of this compound.37,38 Treatment of this open-angle glaucoma is generally medical, although silicone removal may be required. Although some studies have confirmed the presence of emulsified oil in the anterior chamber as a strong predictor of pressure elevation, others do not, suggesting that multiple factors are at play.39,40,41,42 Often this can only be seen on gonioscopy, and removal of the oil, if possible, is beneficial in some cases. Sometimes an expected pressure elevation that would otherwise occur may be muted by concomitant cyclitic membrane formation or hypotony associated with recurrent retinal detachment. The use of shunt procedures in this difficult glaucoma has led to the unusual complication of silicone oil escape into the subconjunctival space43,44 (Fig. 7). Whether placed superiorly or inferiorly, shunts should probably be avoided in these eyes unless the silicone oil can first be safely removed. Transscleral diode laser cyclophotocoagulation has been proven to be an excellent noninvasive option in treating these eyes for recalcitrant pressure elevation.45,46
|
|
|
Intravitreal gas has become a standard part of complex and routine retinal repairs. The appropriate use of intraocular long-acting gas tamponades requires a thorough knowledge of the time-expansion characteristics of the chosen gas (sulfur hexafluoride; perfluoropropane) as well as the clinical situation (partial or total fill of the vitreous cavity).47 Sulfur hexafluoride has a peak expansion 6 to 12 hours after injection. A 20% mixture usually represents the concentration that will not expand in volume when a total fill of the vitreous cavity is performed. Perfluoropropane expands acutely over a similar time frame. A 14% mixture with room air represents the “safe” nonexpansile percentage when the total volume is replaced (Fig. 8).
|
OTHER TRABECULAR MESHWORK INSULTS
Foreign Body Siderosis
Long periods of exposure of intraocular toxic metals such as iron can cause damage to the cells of the trabecular meshwork. Thus, an open-angle glaucoma can ensue that may be recalcitrant to treatment. The unilaterality may be confusing, particularly when the previous ocular history is unknown and the foreign body is occult (Fig. 9).48
Intravitreal Steroids
Intravitreal steroids,49 both depot and the newer delayed release implants,50 have been proposed for the treatment of recalcitrant macular edema or exudation from diabetes, uveitis, macular degeneration, and other conditions.51
As might be expected, these have been found to cause clinically significant pressure elevations in some individuals.52,53,54 As such, intravitreal steroid treatment mandates careful monitoring of intraocular pressure in these patients.
RAISED EPISCLERAL VENOUS PRESSURE
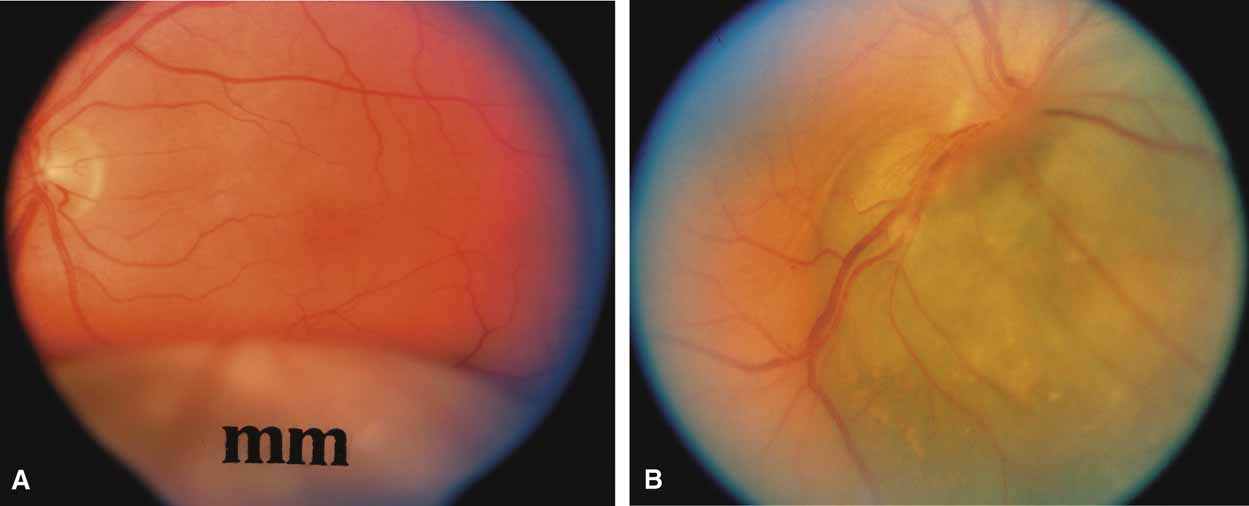
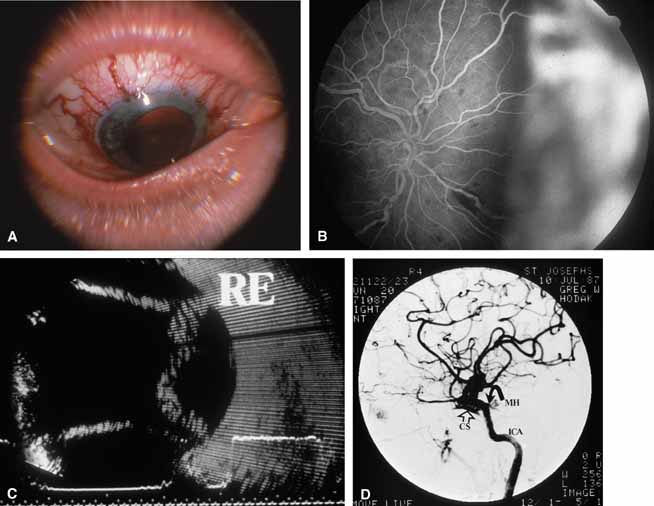
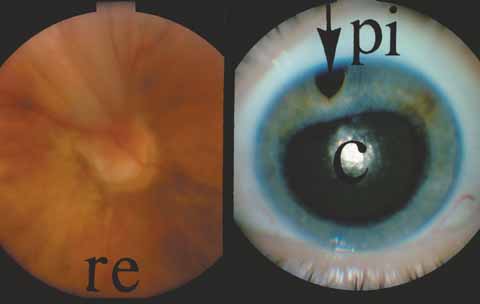
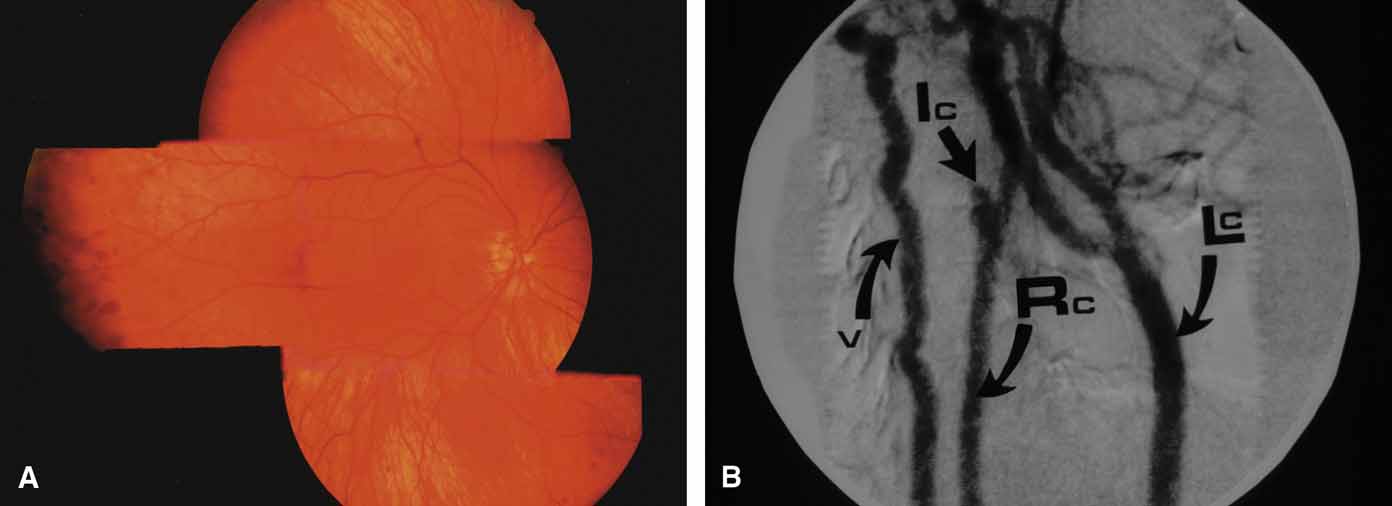
A review of the aqueous blood microcirculation of the eye (Fig. 10) explains how raised venous pressure can affect both retinal vessels and intraocular pressure. From Schlemm's canal, aqueous traverses the intrascleral emissary channels (aqueous veins of Ascher) to the episcleral plexus and then the long ciliary venous vessels. The long ciliary veins, as well as the vortex veins, empty into the ophthalmic vein before entering the cavernous sinus. Increases in episcleral venous backpressure theoretically contribute to a 1:1 mm Hg rise of measured intraocular pressure. The clinical result of an acute rise in external venous pressure may include choroidal effusion, elevated intraocular pressure with blood in Schlemm's canal (Fig. 11), and retinal vein obstruction with intraretinal hemorrhages in a central vein obstruction pattern with or without prominent swelling of the optic nerve. Chronic elevations of venous pressure may permanently damage trabecular meshwork, impairing outflow facility, and result in a chronic open-angle glaucoma.
Superior Vena Cava Syndrome
Obstruction of the venous inflow to the heart may be due to mediastinal tumors, aortic aneurysms, goiters, and enlarged hilar lymph nodes among other causes. A central retinal vein obstruction picture results with engorgement of the retinal veins, peripapillary retinal edema, and elevated intraocular pressure.55 The pressure becomes elevated in the supine position. Correction of the underlying obstruction is ideal, but topical pressure medications may be somewhat effective.
Carotid–Cavernous Fistula
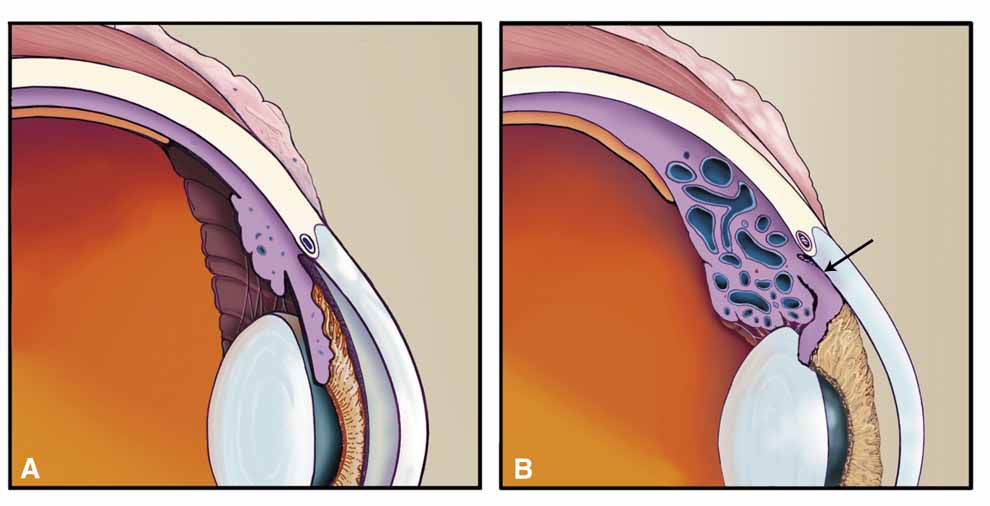
A true shunt between the carotid and cavernous sinusoidal venous system (high-flow type) occurs in 25% of cases and usually results from trauma.56 The more common situation (75% of cases) is a vascular shunt between a dural branch of the external or internal carotid (usually meningohypophyseal branch) and the cavernous sinus (low-flow type).56 There is some evidence that vestigial dural shunts may be congenital and expand when an obstruction occurs in the transverse and sagittal venous drainage systems of the brain.56 The net effect is a direct transmittal of arterial pressure to the retinal and choroidal venous system, which may produce several syndromes. Recent work involving more precise radiologic studies suggests that the specific clinical presentation is determined by the exact location of the vascular anomaly.57 Ocular hypoxia produced by reduced flow may lead to iris neovascularization and glaucoma.58 The more common cause of elevated intraocular pressure is chronic venous pressure elevation that results in permanent damage to the trabecular meshwork. Rarely, vortex venous backpressure may produce choroidal edema, effusion, rotation of the ciliary body, and a congestive glaucoma (Fig. 12).59,60 High- and low-flow arteriovenous shunts pose no threat to human life but have been the object of prodigious surgical efforts. The indications for the surgery should remain intractable eye pain or threatened loss of the eye or other neurological deficits. Shunt flow may be reduced by arterial closure, direct shunt closure, or venous ligation. All these methods, except direct shunt closure, may exacerbate hypoxia and induce iris neovascularization.61
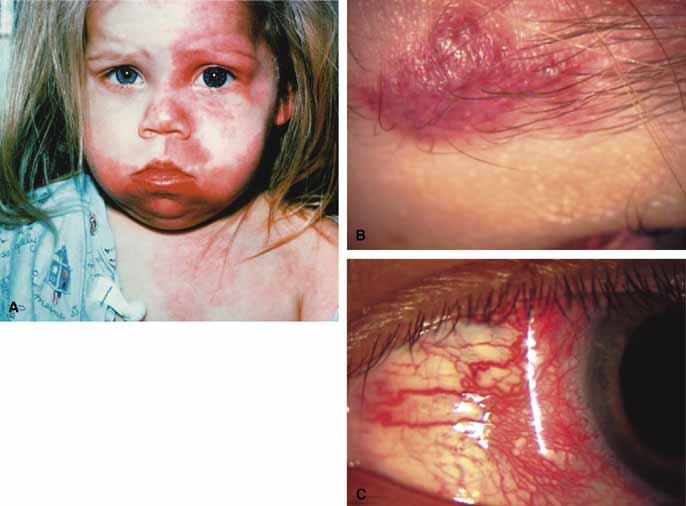
Sturge-Weber Syndrome
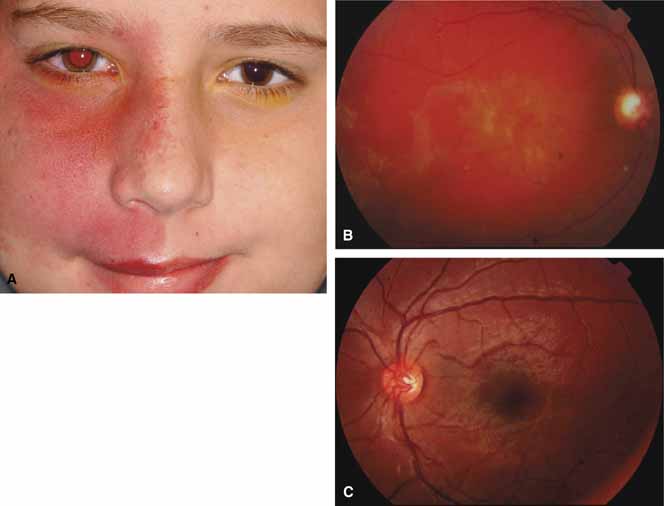
The Sturge-Weber Syndrome is a sporadically occurring phakomatosis that consists of a diffuse choroidal hemangioma, facial nevus flammeus (dermal angioma) (Fig. 13), meningeal angioma with “tram-track” calcification of the brain, and an ipsilateral glaucoma often seen when the dermal angioma involves the upper lid (Fig. 14). The glaucoma may present in infancy or the late teens. In infancy, the glaucoma mechanism may be similar to congenital glaucoma (abnormal trabecular bands) and differ from the later-onset form, which may result from elevated episcleral venous pressure.62 Phelps63 demonstrated elevated episcleral venous pressure in this syndrome and related the extent of the episcleral angioma (often more obvious at surgery when conjunctiva was reflected) to the severity of the glaucoma. Recent work demonstrated thickening of the trabecular beams with abnormalities of the outflow structures, possibly induced by the abnormal vasculature present during embryogenesis.64 Regardless of the exact mechanism, most authorities agree that standard filtration methods carry a high risk of expulsive choroidal hemorrhage. If filtra-tion surgery is contemplated, Bellows and co-workers65 recommend prophylactic sclerostomies prior to decompression of the eye to avoid intraoperative choroidal detachments that may occur in these patients, even when choroidal hemangiomas are not present. Meticulous flap closure that avoids hypotony is the mainstay to preventing choroidal detachments.66 Preoperative scatter laser photocoagulation over the hemangioma, even in the absence of serous retinal detachment, may also reduce the risk of choroidal exudation prior to filtration surgery.66