TRANSIENT POSTOPERATIVE INTRAOCULAR PRESSURE ELEVATION
In the immediate postoperative period, a transient IOP elevation may accompany uncomplicated cataract extraction. Both ICCE23,24 and ECCE25,26 can be associated with significant and rapid changes in IOP after surgery. As stated previously, the incidence is similar between ICCE and ECCE.
The majority of early postoperative conditions are transient and related to outflow obstruction, but chronically elevated IOP may be a sequela of these early postoperative conditions.
Acute Infection
Endophthalmitis can cause an early IOP elevation, and its presence should be considered in the immediate postoperative period in patients with elevated IOP and inflammation. (A complete discussion of postoperative endophthalmitis can be found elsewhere in these volumes.)
Bacterial infection should be suspected whenever IOP elevation is associated with pain, decreased vision, and both intraocular and extraocular inflammation within 1 to 7 days after cataract extraction. Appropriate clinical and laboratory evaluation should be instituted.
MANAGEMENT. Attention to treatment of the infection takes precedence to managing the IOP. The mechanism of IOP elevation is most likely secondary to outflow obstruction from inflammatory cells and debris, and appropriate antimicrobial treatment should aid in clearing this particulate load. If the IOP remains elevated, other underlying mechanisms of IOP elevation should be looked for, identified, and treated as outlined in the remainder of this chapter.
Viscoelastic Substances
The transition from ICCE to ECCE has lead to a transition in postoperative complications. In particular, zonulolytic glaucoma induced by alpha-chymotrypsin has been replaced by transient early IOP elevation associated with viscoelastic substances, particularly sodium hyaluronate (Healon).27–29
IOP elevation can fluctuate as high as 60 mmHg and occurs by the first postoperative day.30 Spontaneous resolution can occur within 48 to 72 hours. Alternative viscoelastic substances, such as chondroitin sulfate and hyaluronic acid (Viscoat), have had similar postoperative IOP elevation problems.31 Methylcellulose 1% to 2% and hydroxypropyl methylcellulose 2% appear to offer some protection to corneal endothelium without raising the IOP.32
Mechanical obstruction of the trabecular meshwork with reduced outflow facility is the most likely primary mechanism of elevated IOP for all the viscoelastic agents.29 In enucleated human eyes, the outflow facility was decreased by 65%.33 Glasser and co-workers28 reported a decrease in postoperative IOP elevation after anterior chamber irrigation of sodium hyaluronate, but Stamper and associates34 found that aspiration of sodium hyaluronate did not significantly reduce the incidence of IOP elevation. Outflow facility in enucleated human eyes was not affected by either irrigation or aspiration of the sodium hyaluronate; it was improved by anterior chamber irrigation with hyaluronidase.
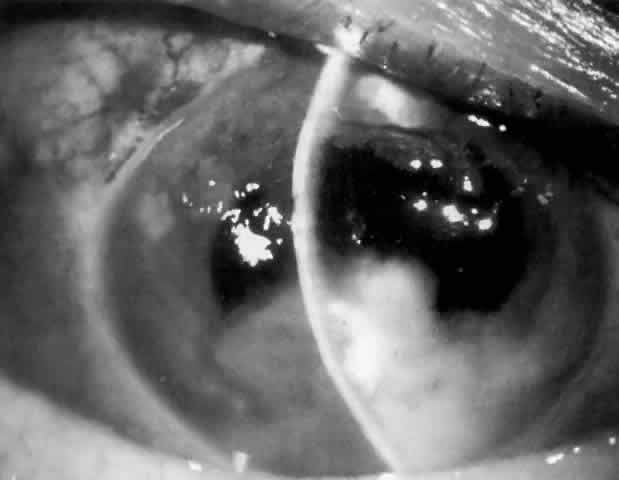
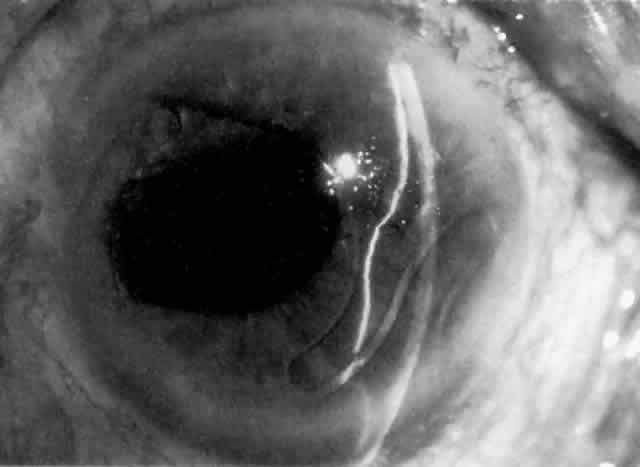
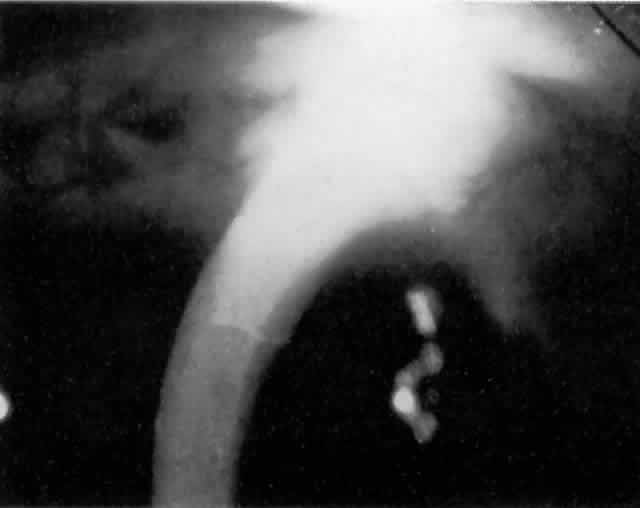
MANAGEMENT. The postoperative IOP elevation that occurs with the use of viscoelastic agents is exacerbated by cortical remnants and blood (Fig. 1). Careful cortical cleanup and aspiration of the viscoelastic agent may reduce the degree of IOP elevation. Prophylactic treatment of all patients (particularly those with preexistent glaucoma) with carbonic anhydrase inhibitors or beta-adrenergic blockers may decrease the incidence or severity of postoperative IOP elevation.35–38
Hyphema
Most postoperative hemorrhages associated with cataract extraction or combined procedures are self-limited. The source of the hemorrhage can be the edge of the corneoscleral wound or the iridectomy. Unless there is a significant amount of blood, debris, or the presence of a viscoelastic substance in the anterior chamber, these early postoperative hyphemas are asymptomatic and not associated with elevated IOP. The mechanism of IOP elevation is obstruction of the trabecular meshwork by one or more of the following: blood, debris, macrophage, and clot. These substances are usually resorbed spontaneously without treatment.
MANAGEMENT. Unless resorption is delayed by excessive debris or compromised preexistent outflow, medical management is employed. Pharmacologic agents used are carbonic anhydrase inhibitors or beta-adrenergic blockers. To minimize iris movement and treat the often attendant intraocular inflammation, cycloplegics and corticosteroids can be of benefit. Surgical evacuation is necessary only if the glaucoma is unresponsive to tolerated medical therapy or there is risk of corneal blood staining.
Inflammation and Lens Particle Glaucoma
Cellular and chemical mediators of inflammation decrease trabecular meshwork function and outflow by a variety of mechanisms and in varying degrees of severity. Both cellular and particulate inflammatory debris can clog trabecular meshwork and compromise outflow.39,40 Additionally, the increased aqueous protein concentration caused by the breakdown of the blood-aqueous barrier also contributes to early postoperative IOP elevation. Each of these inflammatory components are further exacerbated by the presence of any residual viscoelastic substance in the anterior chamber.23 Contrary to decreasing outflow facility, the role that prostaglandins play in postoperative IOP elevation may relate to their effect on increasing aqueous production.
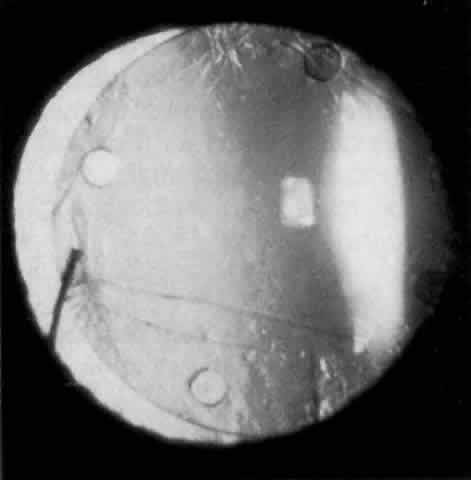
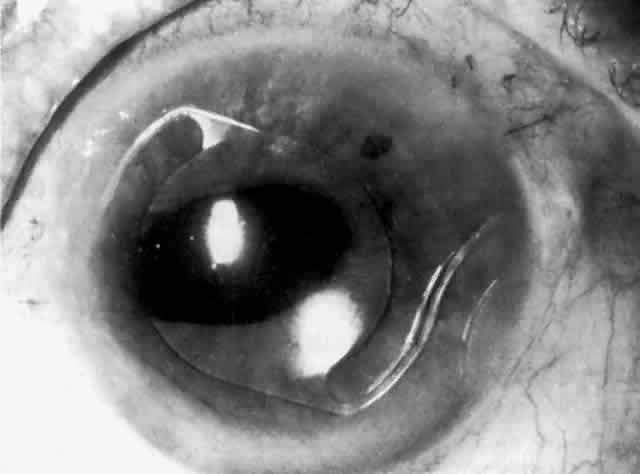
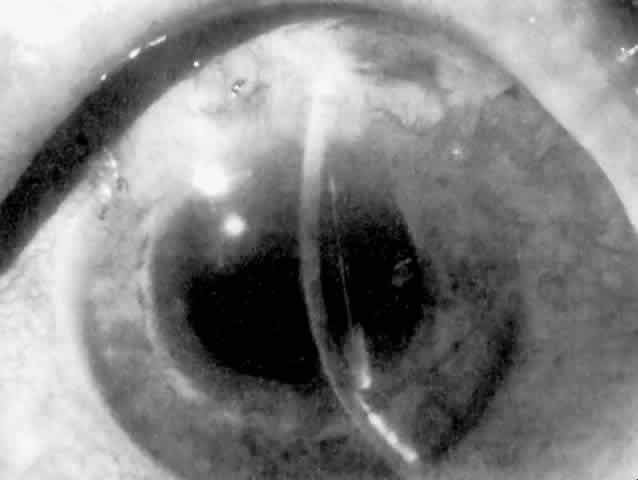
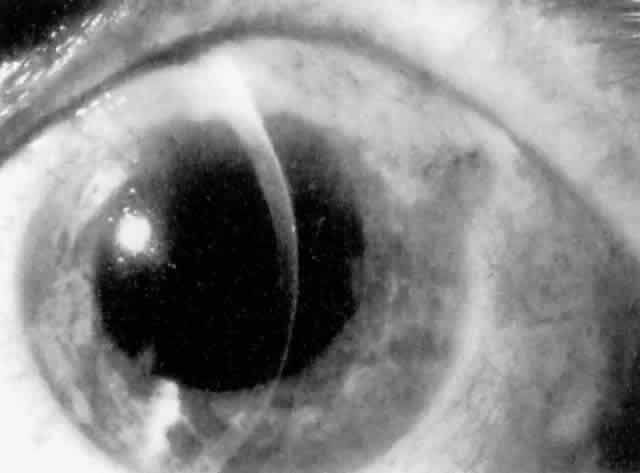
Cortical lens fragments retained in either the anterior chamber or the vitreous can also obstruct the trabecular meshwork in the form of free lens particles or macrophages swollen with lens material (Figs. 2 and 3). Glaucoma does not occur in all eyes that contain cortical remnants; the inflammatory response may be more pronounced and prolonged in eyes containing a higher amount of lens material. When inflammation is marked, keratic precipitates and sometimes a hypopyon may be present. Distinction between this sterile inflammatory endophthalmitis and infectious endophthalmitis can be difficult and may depend on the initial response to therapy. The presence or absence of IOP elevation is not helpful in making this distinction because IOP may be normal or elevated in both situations.
|
MANAGEMENT. Inflammation is likely to occur to some degree after cataract extraction. The degree to which this will affect postoperative IOP depends to some extent on preexistent trabecular meshwork function and the severity of the inflammatory response. In general, treatment is aimed at controlling the inflammation with corticosteroids. Aspirin and nonsteroidal anti-inflammatory agents (e.g., indomethacin) may also help to control postoperative inflammation.
Management of elevated IOP relies on treatment with beta-adrenergic blockers and carbonic anhydrase inhibitors. Because of the disruption of the blood-aqueous barrier, hyperosmotic agents are of limited utility in the postoperative patient with inflammation, and miotics should be avoided because they further disturb the blood-aqueous barrier and promote formation of posterior synechiae. Mydriatics or cycloplegics to decrease iris movement and prevent formation of posterior synechiae are recommended if inflammation is marked. If posterior synechiae do develop in the inflamed postoperative eye, attempts should be made to break these with dilation to avoid pupillary block as a result of a secluded pupil. If pharmacologic mydriasis is unsuccessful, peripupillary iridoplasty may achieve photomydriasis.41 Once iris bombé does develop, however, a laser iridotomy is indicated if vigorous mydriasis is unsuccessful.
Lens particle glaucoma treatment is similarly aimed at the underlying inflammation. The glaucoma usually resolves as the retained cortical material resorbs and inflammation decreases. Beta-adrenergic blockers and carbonic anhydrase inhibitors are of temporary help in controlling this transient IOP elevation. If spontaneous resorption is prolonged or medical therapy either fails or is not well tolerated, surgical removal of the residual cortex is necessary.
Vitreous in the Anterior Chamber
Anterior chamber vitreous prolapse can occur after an ICCE or after inadvertent rupture or dialysis of the posterior capsule during ECCE. In the case of phacoemulsification or planned ECCE, vitreous prolapse is often associated with other IOP-provoking elements, such as inflammatory debris or blood.42,43 The mechanism of open-angle postoperative glaucoma in this setting may be related to the degree of inflammation and blood in the anterior chamber; it may also be complicated by a direct obstruction of the trabecular meshwork by vitreous fibrils.44
MANAGEMENT. Management is most often medical rather than surgical. Beta-adrenergic blockers and carbonic anhydrase inhibitors are used to control the IOP elevation. Cycloplegics combined with hyperosmotics may help draw the vitreous from the anterior chamber angle. Many cases spontaneously resolve in several months as the vitreous recondenses and retracts from the angle. Anterior vitrectomy may be required if medical management is unable to control the IOP.
Trabecular Meshwork Dysfunction and Anterior Chamber Angle Distortion
The gonioscopic appearance of the corneoscleral incision after cataract extraction has been well described by Kirsch and co-workers.45,46 A white ridge resembling an “inverted snow bank” lines the inner margin of the corneoscleral incision. For approximately the first 2 weeks after cataract extraction, this ridge is seen to obscure the trabecular meshwork and to distort the anterior chamber angle. The decrease in outflow facility improves with resolution of the corneal scleral ledge.47 Clear corneal incisions do not develop this healing ridge. In one series of 95 cataract extractions, early postoperative IOP elevation with evidence of this inner ridge developed in 23% of cases involving limbal incisions; postoperative IOP was unaffected in cases involving cataract extractions with corneal incisions.48 Theories regarding the pathogenesis of this incisional ridge range from tight corneoscleral sutures to corneal stromal and trabecular meshwork edema.49
MANAGEMENT. Management of reduced outflow follows the route taken for inflammation, since some portion of this reduced outflow may recover as the inflammation resolves.
Alpha-Chymotrypsin
Alpha-chymotrypsin is an enzyme with a selective propensity to lyse lens zonules. The glaucoma induced by alpha-chymotrypsin has been referred to as zonulolytic glaucoma. In two studies on the effects of alpha-chymotrypsin on IOP, it was found that IOP elevation usually occurred 2 to 5 days after ICCE in 27% of patients in whom alphachymotrypsin was used for zonulolysis.50,51 IOP elevation is more common in patients with preexisting open-angle glaucoma. Tonographic studies have demonstrated a transient decrease in aqueous outflow facility.52–54 No long-term alteration in outflow has been demonstrated in eyes with zonulolytic glaucoma unless there was preexistent glaucoma. Histologic studies of animal eyes have suggested that accumulation of lens zonule fragments in the trabecular meshwork produces a transient outflow obstruction.55–57
MANAGEMENT. Zonulolytic glaucoma resolves within 48 to 72 hours. Medical therapy with beta-adrenergic blockers and carbonic anhydrase inhibitors is usually adequate. Preoperative or intraoperative administration of acetylcholine, mannitol, pilocarpine, or corticosteroids does not prevent zonulolytic glaucoma.58 Intraoperative irrigation of the anterior chamber to remove zonular fragments does not affect the degree of elevated IOP, but the use of minimum volumes of the enzyme can be effective in decreasing the risk of its occurrence.
PERSISTENT OR LATE POSTOPERATIVE INTRAOCULAR PRESSURE ELEVATION
Irreversible Trabecular Meshwork Damage
After cataract extraction, the IOP usually returns to normal within 1 week. If, however, the patient has a history of dysfunction of the trabecular meshwork (e.g., preexistent glaucoma) this resolution may be prolonged. In any patient, the IOP elevation can become sustained or permanent as a result of any of the multiple insults of cataract surgery on the trabecular meshwork.
MANAGEMENT. The chronic glaucoma in aphakia or pseudophakia should initially be evaluated for other possible treatable causes of sustained IOP elevation (e.g., steroids, recurrent hemorrhage, persistent inflammation, angle closure). Therapy should initially be directed at these problems if identified.
Patients whose persistent IOP elevation is not directly treatable should be managed medically. Miotics, beta-adrenergic blockers, and carbonic anhydrase inhibitors may be effective. Epinephrine compounds have been associated with macular edema in pseudophakic and aphakic patients and therefore should be used only with extreme caution.59 Postoperative laser trabeculoplasty may be effective, but it is generally considered more successful when performed before cataract extraction.10,11 Surgery is reserved for patients who cannot be managed on maximum tolerable medical therapy.
Therefore, management follows the route taken for simple open-angle glaucoma, with the exception of filtration surgery. Filtering surgery for aphakia and pseudophakia has a lower success rate than that reported for phakic eyes. The Multicenter 5-Fluorouracil Filtering Surgery Study reported that 28% of eyes that received postoperative subconjunctival 5-fluorouracil required reoperation during the first 3 postoperative years for IOP control, compared with 60% of the standard-treatment group. Antimetabolites, 5-fluorouracil, and mitomycin C have become proven adjuncts in glaucoma surgery for aphakia and pseudophakia.60–65
Persistent Inflammation
Persistent inflammation after cataract surgery may indicate one of several possibilities: low-grade infection, IOL-related inflammation, residual lens material, or epithelial downgrowth. (See previous section on Inflammation and Lens Particle Glaucoma for a discussion of the effects of residual lens material.) Chronic low-grade infections can include fungal causes; however, these are rare and are associated with marked inflammation. A persistent low-grade infection associated with persistent, subtle inflammation may be due to Propionibacterium acnes infection. This bacterium may be sequestered in residual cortex, and since anterior chamber taps and cultures are often negative, the diagnosis may be difficult to confirm.
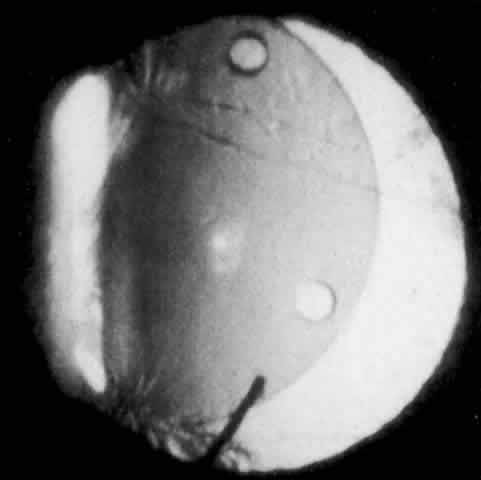
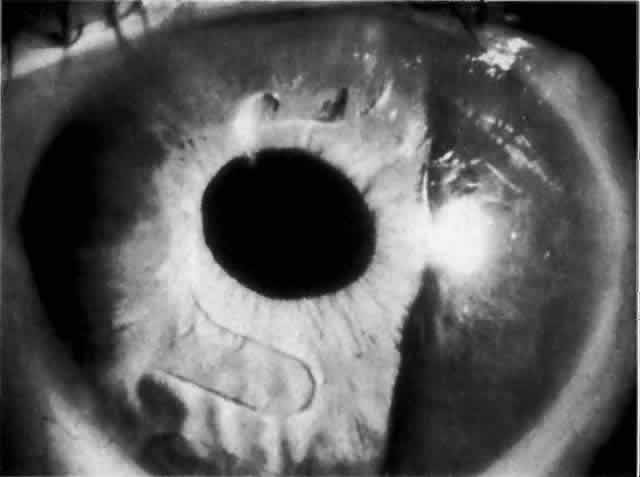
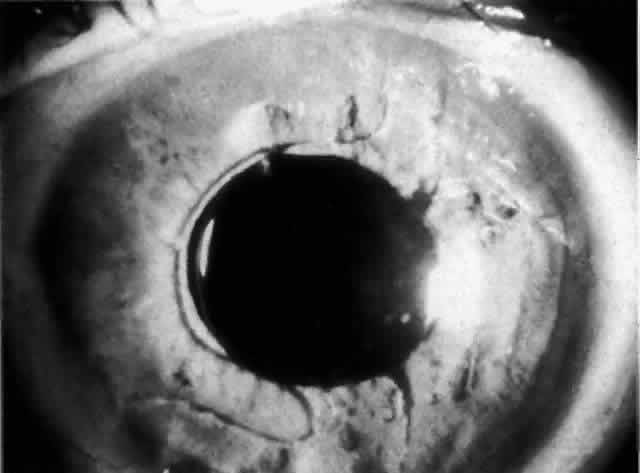
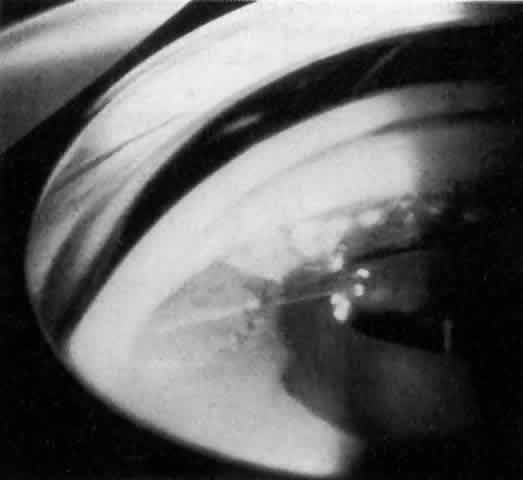
IOL-related inflammation is more commonly associated with some styles of lens implants than with others. Position of the lens or foreign material deposited on the lens during manufacture can also account for persistent inflammation. When the inflammation is associated with hyphema, it is termed UGH syndrome, which involves the triad of uveitis, glaucoma, and hyphema. UGH syndrome occurred more frequently in the 1970s, when iris-fixed lenses were commonly used.66,67 Today it is more often associated with anterior chamber lenses and is less likely to occur with posterior chamber lenses, although this has been reported.68,69 The mechanism is believed to be contact of the surface of the lens with the iris, causing mechanical irritation that may be enhanced by a poorly positioned or mobile lens implant. The loose posterior chamber lens may initially exhibit release of pigment due to friction on the posterior surface of the iris (pseudopigmentary glaucoma). This “windshield-wiper” effect alone can produce enough pigment to provoke an increase in IOP; however, it is the erosion into vascular tissue that results in hemorrhage, hyphema, and inflammation (Figs. 4 and 5).
|
|
MANAGEMENT. Treatment of a P. acnes infection depends on the organism's being found or suspected when all taps and cultures are negative for other possible organisms. Removal of residual lens and capsule will usually resolve the situation, but if peripheral anterior synechiae have formed as a result of the persistent inflammation, the IOP elevation may require standard glaucoma management. IOL-related inflammation is also often reversible once the offending implant is removed. Some mild cases may be managed by minimizing iris movement against the lens caused by chronic use of mydriatics or cycloplegics. The IOP elevation will usually resolve unless persistent outflow obstruction exists in the form of synechiae closure or direct injury to the angle by the lens implant haptics.
Recurrent Hyphema
Hyphema without neovascularization of the iris can occur months or years after cataract extraction. This usually indicates vascularization of the cataract wound, Swan's syndrome,69 or erosion of a uveal vessel by an implant. Gonioscopic examinations can reveal a localized clot or vessels along the cataract incision site. These episodes are often transient and resolve with minimal treatment. The exception is IOL-related erosion of a uveal vessel, which can result in UGH syndrome.
MANAGEMENT. Management of each episode of acute IOP elevation in recurrent hyphema should be similar to that employed for hyphema in the immediate postoperative period. Treatment approaches include cycloplegia, corticosteroids, and aqueous suppressants. On rare occasions, if vascularization of the cataract wound is clear, argon laser ablation can be attempted. Treatment for IOL erosion consists of either cycloplegia to minimize iris movement or removal of the lens. It should be noted, however, that lens exchange or removal alone will not be sufficient to control the IOP when there is sufficient preexisting synechiae formation or trauma to the trabecular meshwork, in which case concomitant glaucoma surgery may need to be performed.
Ghost Cell Glaucoma
Ghost cell glaucoma can occur in both aphakic and pseudophakic eyes in which there is a vitreous hemorrhage and a disrupted vitreous face. Because of their reduced pliability compared with fresh red blood cells, tan or khaki cells obstruct outflow channels. IOP elevation, while self-limited, may persist longer than expected because of the large reservoir in the vitreous cavity. IOL-induced hemorrhage releasing blood cells into the vitreous cavity has resulted in ghost cell glaucoma, which can recur.70
MANAGEMENT. Ghost cell glaucoma is often self-limited with the exception of IOL-induced hemorrhage. Management requires aqueous suppressants, cycloplegia, and frequent examinations. Vitrectomy and anterior chamber irrigation may be necessary to eliminate the reservoir of ghost cells. Removing or repositioning the offending IOL implant may be combined with this procedure if the IOL is believed to have been the source of hemorrhage.
Persistent Release of Pigment: Pseudopigmentary Glaucoma
As mentioned in the Persistent Inflammation section, pseudopigmentary glaucoma is in many ways similar to the persistent inflammation that results from an IOL implant. The mechanism is believed to be contact of the surface of the lens with the iris. In persistent inflammation and UGH syndrome, typically either an anterior chamber lens or an iris-fixed lens is the culprit. With respect to pseudopigmentary glaucoma, however, the lens used is more likely to be a posterior chamber lens with the haptics (either one or both) positioned in the sulcus. In this way, the pigmented surface of the iris moves back and forth across the edge of the IOL, promoting release of pigment into the anterior chamber (see Figs. 4 and 5). The mechanism of IOP elevation is outflow obstruction from the excessive amounts of pigment granules and cell debris in the trabecular meshwork.
MANAGEMENT. Mild cases of pseudopigmentary glaucoma may be managed with aqueous suppressants and either mydriatics or miotics to minimize iris movement. Corticosteroids are not indicated. More severe cases may require IOL removal or replacement.