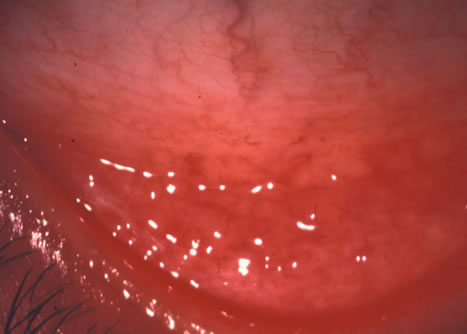
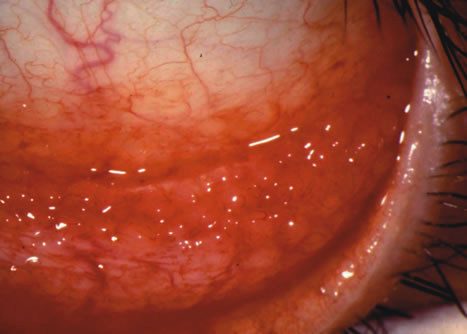
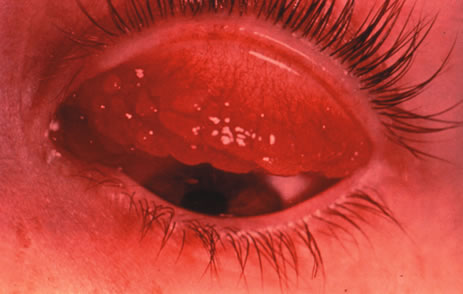
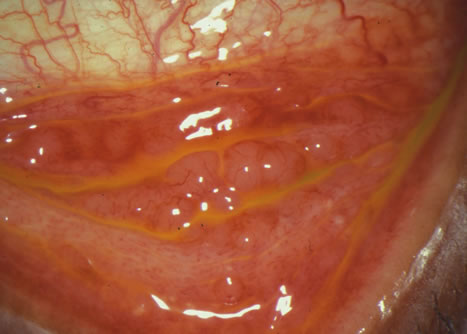
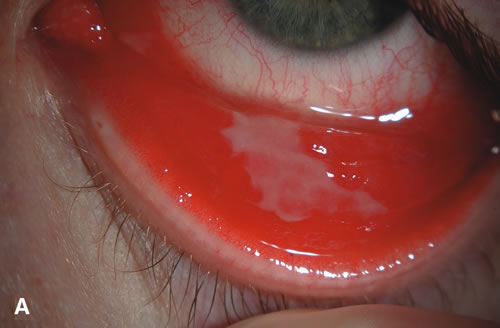
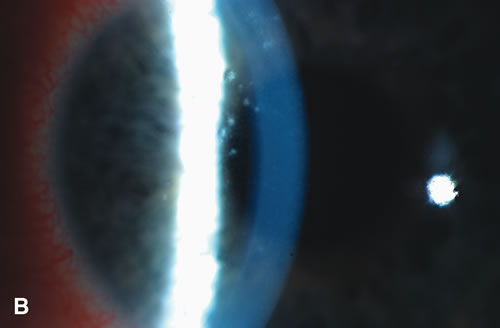
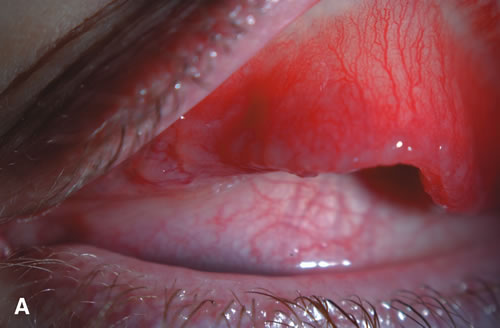
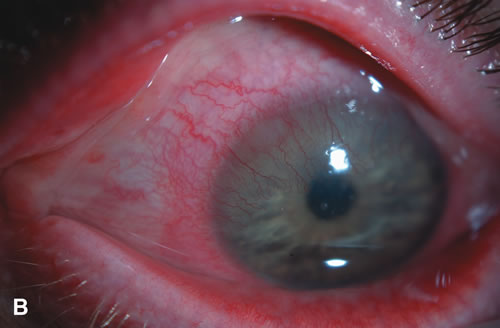
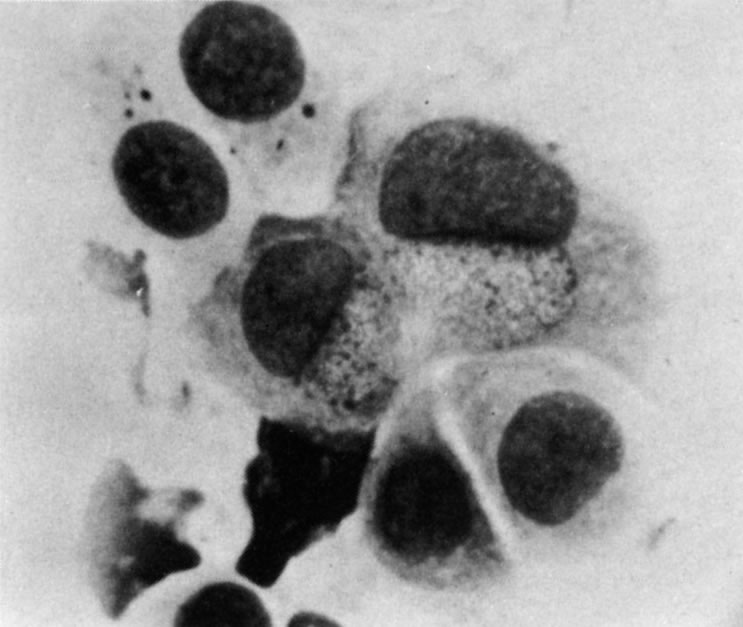
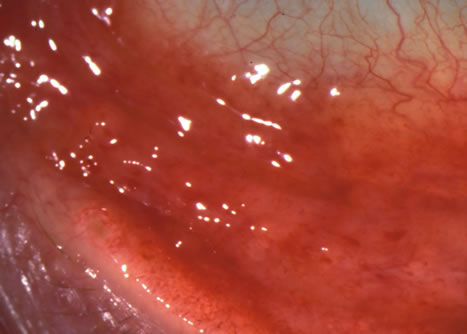
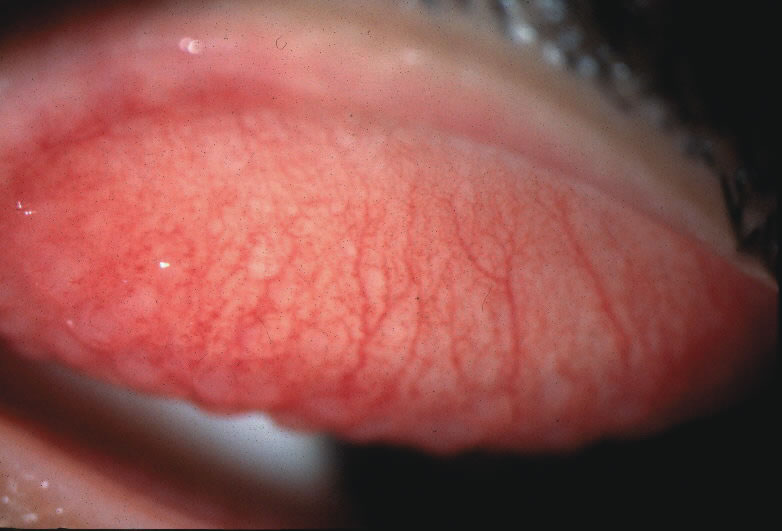
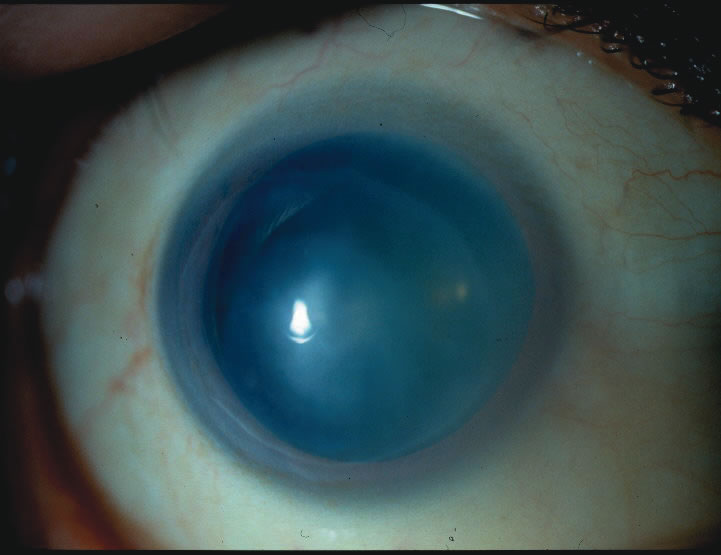
1. Duke-Elder S: System of Ophthalmology: Diseases of the Outer Eye. Vol 8, part 1. Diseases of the Conjunctiva and Associated Diseases of the Corneal Epithelium. London, Henry Kimpton, 1965 2. Yanoff M, Fine BS: Conjunctiva. In: Ocular Pathology: A Text and Atlas. Philadelphia, Lippincott and Co., 1989 3. Chandler JW, Gillettte TE: Immunologic defense mechanisms of the ocular surface. Ophthalmology 90:585, 1983 4. Belfort R Jr, Mendes NF: Identification of T and B lymphocytes in the human conjunctiva and lacrimal
gland in ocular disease. Br J Ophthalmol 64:217, 1980 5. Rowe WP, Huebner RJ, Gilmore LK, et al: Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous
degeneration in tissue culture. Proc Soc Exp Soc Med 84:570, 1953 6. De Jong JC, Wermenbol AG, Verweij-Uijterwal MW, et al: Adenoviruses from human immunodeficiency virus–infected individuals
including 2 strains that represent new candidate serotypes Ad50 and
Ad51 of species B1 and D respectively. J Clin Microbiol 37:3940, 1999 7. Toogod CI, Murali R, Bennett RM, et al: The adenovirus type 40 hexon: sequence predicted structure and relationship
to other adenovirus hexons. J Gen Virol 70:3203, 1989 8. Fox JP, Hall CE, Cooney MK: The Seattle virus watch. Observations of adenovirus infections. Am J Epidemiol 105:362, 1977 9. Jernigan JA, Lowry FG, Hayden SA, et al: Adenovirus type 8 epidemic keratoconjunctivitis in an eye clinic: risk
factors and control. J Infect Dis 167:1307, 1993 10. Nauheim RC, Romanowski T, Araullo-Cruz RP, et al: Prolonged recoverability of dessicated adenovirus type 19 from various
surfaces. Ophthalmology 97:1450, 1990 11. Warren D, Nelson JA, Farrar E, et al: A large outbreak of epidemic keratoconjunctivitis: problems in controlling
nosocomial spread. J Infect Dis 160:938, 1989 12. Fuchs E. Keratitis puncta superficialis. Wien Klin Wochenschr 44:837, 1889 13. , Jawetz E, Kimura SJ, Hanna L, et al: New type of APC virus from epidemic keratoconjunctivitis. Science 122:1190, 1955 14. Lund OE, Stefani FH: Corneal histology after epidemic keratoconjunctivitis. Arch Ophthalmol 96:2085, 1978 15. Gunther R: Pathologisch-anatomischer Befund einer Hornhaut bei Keratitis epidemica. Klin Monatsbl Augenheilkd 103:309, 1939 16. Sundmacher R, Engelskirchen U: Zum Problem der rezidevierenden und persistierenden Nummuli nach Keratoconjunctivitis
epidemica. Klin Monatsbl Augenheilkd 198:550, 1991 17. De Clercq E: Therapeutic potential of HPMPC as an antiviral drug. Rev Med Virol 3:85, 1993 18. Gordon YJ, Romanowski EG, Araullo-Cruz T: Topical HPMPC inhibits adenovirus type 5 in the New Zealand rabbit ocular
replication model. Invest Ophthalmol Vis Sci 35:4135, 1994 19. Hillenkamp J, Reinhard T, Ross RS, et al: The effects of cidofovir 1% with and without cyclosporin A 1% as a topical
treatment of acute keratoconjunctivitis: a controlled clinical pilot
study. Ophthalmology 109:845, 2002 20. Dawson CR, Darrell R: Infections due to adenovirus type 8 in the United States. An outbreak of
epidemic keratoconjunctivitis originating in a physician's office. N Eng J Med 268:1031, 1963 21. Ford E, Nelson KE, Warren D: Epidemiology of epidemic keratoconjunctivitis. Epidemiol Rev 9:244, 1987 22. Azar MJ, Dhaliwal DK, Bower KS, et al: Possible consequences of shaking hands with your patients with epidemic
keratoconjunctivitis. Am J Ophthalmol 121:711, 1996 23. Nauheim RC, Romanowski T, Araullo-Cruz RP, et al: Prolonged recoverability of desiccated adenovirus type 19 form various
surfaces. Ophthalmology 97:1450, 1990 24. Dawson CR, Hanna L, Wood TR, et al: Adenovirus type 8 keratoconjunctivitis in the United States. Am J Ophthalmol 69:473, 1970 25. Darougar S, Quinlan MP, Gibson JA, et al: Epidemic keratoconjunctivitis and chronic papillary conjunctivitis in London
due to adenovirus type 19. Br J Ophthalmol 61:76, 1977 26. Ford E, Nelson KE, Warren D: Epidemiology of epidemic keratoconjunctivitis. Epidemiol Rev 9:244, 1987 27. D'Angelo LJ, Hierholzer JC, Keenlyside RA, et al: Pharyngoconjunctival fever caused by adenovirus type 4: report of a swimming
pool–related outbreak with recovery of virus from pool water. J Infect Dis 140:42, 1979 28. Deckard PS, Bergstrom TJ: Rubeola keratitis. Ophthalmology 88:810, 1981 29. Sandford-Smith JH, Whittle HC: Corneal ulceration following measles in Nigerian children. Br J Ophthalmol 63:720, 1979 30. Foster A, Sommer A: Corneal ulceration, measles and childhood blindness in Tanzania. Br J Ophthalmol 71:331, 1987 31. Riffenburgh RS: Ocular manifestations of mumps. Arch Ophthal 66:739, 1961 32. Meyer RF, Sullivan JH Oh JO: Mumps conjunctivitis. Am J Ophthalmol 78:1022, 1974 33. Hales RH, Ostler BH: Newcastle disease conjunctivitis with subepithelial infiltrates. Br J Ophthalmol 57:694, 1973 34. Hara J, Fujimoto F, Ishibashi T, et al: Ocular manifestations of the 1976 rubella epidemic in Japan. Am J Ophthalmol 87:642, 1979 35. WHO Weekly Epidemiological Record56:293, 1981 36. Chang CH, Sheu MM, Lin KH, et al: Hemorrhagic viral keratoconjunctivitis in Taiwan caused by adenovirus types 19 and 37: application of polymerase chain reaction-restriction fragment
length polymorphism in detecting adenovirus genotypes. Cornea 20:295, 2001 37. Uchio E, Yamazaki K, Ishikawa H, et al: An epidemic of acute haemorrhagic conjunctivitis caused by enterovirus 70 in
Okinawa, Japan, in 1994. Graefes Arch Clin Exp Ophthalmol 237:568, 1999 38. Yin-Murphy M, Goh KT, Phoon MC, et al: A recent epidemic of acute hemorrhagic conjunctivitis. Am J Ophthalmol 116:212, 1993 39. Whitcher JP, Schmidt NJ, Mabrouk R, et al: Acute hemorrhagic conjunctivitis in Tunisia. Report of viral isolations. Arch Ophthalmol 94:51, 1976 40. Chopra A, Rana PV, Narayanaswamy AS, et al: Neurological complications following acute viral conjunctivitis: a new
profile. Trop Geogr Med 38:197, 1986 41. Alphonso E, Friedland B, Hupp S, et al: Neisseria gonorrhoea conjunctivitis: an outbreak during an epidemic of acute hemorrhagic conjunctivitis. JAMA 250:794, 1983 42. Falk ES: Parapoxvirus infection of reindeer and musk ox associated with unusual
human infection. Br J Dermatol 99:647, 1978 43. Freeman G, Bron AJ, Juel-Jensen B: Ocular infection with orf virus. Am J Ophthalmol 97:601, 1984 44. Moore RM Jr: Human Orf in the United States. J Infect Dis 127:731, 1973 45. Glasser DB, Hyndiuk RA: Herpes simplex keratitis. In Tabbara KF, Hyndiuk RA (eds): Infections of the Eye. Boston, Little Brown, 1986 46. Harding SP, Mallinson H, Smith JL, et al: Adult follicular conjunctivitis and neonatal ophthalmia in a Liverpool
eye hospital 1980-84. Eye 1:512, 1987 47. Uchio E, Takeuchi S, Hoh N, et al: Clinical and epidemiological features of acute follicular conjunctivitis
with special reference to that caused by herpes simplex virus type 1. Br J Ophthalmol 84:968, 2000 48. Herpetic Eye Disease Study Group: Acyclovir for the prevention of recurrent
herpes simplex virus eye disease. N Engl J Med 339:300, 1998 49. Matoba AY, Jones DB: Corneal subepithelial infiltrates associated with systemic Epstein-Barr
viral infection. Ophthalmology 94:1669, 1987 50. Ostler HB: Oculogenital disease. Surv Ophthalmol 20:233, 1976 51. Schachter J: Chlamdial infection. N Eng J Med 298:428, 1978 52. Whitcher JP: Chlamydial diseases. In Smolin G, Thoft RA (eds): The Cornea: Scientific Foundations and Clinical Practice.2nd ed. Boston, Little Brown, 1981 53. Ronnerstam R, Persson K, Hansson H, et al: Prevalence of chlamydial eye infection in patients attending an eye clinic, a
VD clinic and in healthy persons. Br J Ophthalmol 69:385, 1985 54. Viswalingham ND, Wishart MS, Woodland RM: Adult chlamydia ophthalmia. Br Med Bull 93:123,1983 55. Brunham RC, Paavonen J, Stevens CE, et al: Mucopurulent cervicitis—the ignored counterpart in women of urethritis
in men. N Eng J Med 311:1, 1984 56. Potts MJ, Paul ID, Roome AP, et al: Rapid diagnosis of Chlamydia trachomatis infection in patients attending an ophthalmic casualty department. Br J Ophthalmol 70:677, 1986 57. Schachter J, Dawson CR: Human Chlamydial Infections. Littleton, MA, PSG,1978 58. Centers for Disease Control and Prevention: 1998 guidelines for treatment
of sexually transmitted diseases. MMWR Recomm Rep 47:1, 1998 59. Francois P, Rouhan D, Hirtz P, et al: Chlamydia trachomatis en pediatrie. Pediatrie 43:101, 1988 60. Harrison HR, English MG, Lee CK, et al: Chlamydia trachomatis infant pneumonitis: comparison with matched controls and other infant
pneumonitis. N Engl J Med 298:702, 1978 61. Talley AR, Garcia-Ferrer F, Laycock KA, et al: Comparative diagnosis of neonatal chlamydial conjunctivitis by polymerase
chain reaction and McCoy cell culture. Am J Ophthalmol 117:50, 1994 62. Beem MO, Saxon EM: Respiratory tract colonization and a distinctive pneumonia syndrome in
infants infected with Chlamydia trachomatis. N Eng J Med 298:306, 1978 63. Thylefors B, Negrel AD, Pararajasegaram R, Dadzie KY: Global data on blindness. Bull World Health Organ 73:115, 1995 64. Courtright P, Sheppard J, Schachter J, et al: Trachoma and blindness in the Nile Delta: current patterns and projections
for the future in the rural Egyptian population. Br J Ophthalmol 73:536, 1989 65. Dawson CR, Daghfous T, Messadi M, et al: Severe endemic trachoma in Tunisia. Br J Ophthalmol 60:245, 1976 66. Jones BR: The prevention of blindness from trachoma. Trans Ophthalmol Soc UK 95:16, 1975 67. West S, Munoz B, Lynch M, et al: Impact of face washing on trachoma in Kongwa, Tanzania. Lancet 345:155, 1995 68. Treharne JD: The community epidemiology of trachoma. Rev Infect Dis 7:760, 1985 69. Grayston JT, Wang S: New knowledge of chlamydiae and the diseases they cause. J Infect Dis 132:87, 1975 70. Abuel-Asrar AM, Gebose K, Missotten L: Immunology of trachomatous conjunctivitis. Bull Soc Belge Ophtalmol 280:73, 2001 71. Darougar S, Jones BR: Trachoma. Br Med Bull 39:117, 1983 72. Bowman RJ, Jatta B, Charn B, et al: Natural history of trachomatous scarring in the Gambia: results of a 12-year
longitudinal followup. Ophthalmology 108:2219, 2001 73. Munoz B, Bobo L, Mkocha H, et al: Incidence of trichiasis in a cohort of women with and without scarring. Int J Epidemiol 28:1167, 1999 74. Munoz B, Aron J, Turner V, et al: Incidence estimates of late stages of trachoma among women in a hyperendemic
area of central Tanzania. Trop Med Int Health 2:1030, 1997 75. Dolin PJ, Faal H, Johnson GJ, et al: Trachoma in the Gambia. Br J Ophthalmol 82:930, 1998 76. Dawson CR, Juster R, Marx R, et al: Limbal disease in trachoma and other ocular chlamydial infections: risk
factors for corneal vascularisation. Eye 3:204, 1989 77. Halberstaedter L, Von Prowazek S: Sur Aetiologie das Trachome. Dtsch Med Wochenschr 33:1285, 1907 78. Taylor HR, Fitch CP, Murillo-Lopez F, et al: The diagnosis and treatment of chlamydial conjunctivitis. Int Ophthalmol 12:95, 1988 79. Marx R: Social factors and trachoma: a review of the literature. Soc Sci Med 29:23, 1989 80. Mabey DCW, Bailey RL, Hutin YJF: The epidemiology and pathogenesis of trachoma. Rev Med Microbiol 3:112, 1992 81. Bobo L, Munoz B, Viscidi R, et al: Diagnosis of Chlamydia trachomatis eye infection in Tanzania by polymerase chain reaction/enzyme immunoassay. Lancet 338:847, 1991 82. Mahdi OS: Impact of host genetics on susceptibility to human Chlamydia trachomatis disease. Br J Biomed Sci 59:128, 2002 83. West S, Munoz B, Lynch M, et al: Impact of face washing on trachoma in Kongwa, Tanzania. Lancet 345:155, 1995 84. Dawson CR, Hanna L, Wood TR, et al: Controlled trials with trisulfapyrimidines in the treatment of chronic
trachoma. J Infect Dis 119:581, 1969 85. Dawson CR, Daghfous T, Hoshiwara I, et al: Trachoma therapy with topical tetracycline and oral erythromycin: a comparative
trial. Bull World Health Organ 60:347, 1982 86. Darougar S, Jones BR, Viswalingam N, et al: Topical therapy of hyperendemic trachoma with rifampacin, oxytetracycline
or spiramycin eye ointment. Br J Ophthalmol 64:37, 1980 87. Schachter J, West SK, Mabey D, et al: Azithromycin in control of trachoma. Lancet 354:6301999 88. Lietman T, Porco T, Dawson C, et al: Global elimination of trachoma: how frequently should we administer mass
chemotherapy?Nat Med 5:492, 1999 89. Fry AM, Jha HC, Lietman TM, et al: Adverse and beneficial secondary effects of mass treatment with azithromycin
to eliminate blindness due to trachoma in Nepal. Clin Infect Dis 35:395, 2002 90. Reacher MH, Munoz B, Alghassany A, et al: A controlled trial of surgery for trichiasis of the upper lid. Arch Ophthalmol 110:667, 1992 91. Bowman RJC, Faal H, Jatta B, et al: Longitudinal study of trachomatous trichiasis in the Gambia: barriers to
acceptance of surgery. Invest Ophthalmol Vis Sci 43:936, 2002 92. Mabey D, Bailey R: Eradication of trachoma worldwide. Br J Ophthalmol 83:1261, 1999 93. Schachter J, Arnstein P, Dawson CR, et al: Human follicular conjunctivitis caused by infection with a psittacosis
agent. Proc Soc Exp Biol Med 127:292, 1968 94. Lietman T, Brooks D, Moncada J, et al: Chronic follicular conjunctivitis associated with Chlamydia psittaci or Chlamydia pneumoniae. Clin Infect Dis 26:1335, 1998 95. Kowalski RP, Harwick JC: Incidence of Moraxellaconjunctivitis infection. Am J Ophthalmol 101:437, 1986 96. Parinaud H: Conjunctivite infectieuse araissant transmise a l'homme par les animaux. Bull Xoc Ophtalmol Paris 2:29, 1889 97. Carithers HA: Cat-scratch disease: an overview based on a study of 1200 patients. Am J Dis Child 139:1124, 1985 98. Cunnigham ET, Keohler JE: Ocular bartonellosis. Am J Ophthalmol 130:340, 2000 99. Madu AA, Mayers M: Ocular manifestation of systemic infections. Curr Opin Ophthalmol 6:88, 1995 100. Labalett P, Bermond D, Vedes V, et al: Cat-scratch disease neuroretinitis diagnosed by a PCR approach. Arch Ophthalmol 132:575, 2001 101. Margileth AM: Antibiotic therapy for cat-scratch disease: clinical study of therapeutic
outcome in 268 patients and a review of the literature. Pediatr Infect Dis J 11:474, 1992 102. Steere AC, Malawista SE, Snydman DR, et al: Lyme arthritis: an epidemic of oligoarticular arthritis in children and
adults in three Connecticut communities. Arthritis Rheum 20:7, 1977 103. Mombaerts IM, Maudgal PC, Knockaert D: Bilateral follicular conjunctivitis as a manifestation of Lyme disease. Am J Ophthalmol 112:96, 1991 104. Orlin SE, Lauffer JL: Lyme disease keratitis. Am J Ophthal 107:678, 1988 105. Fiore PM, Jacobs IH, Goldberg DB: Drug-induced pemphigoid. A spectrum of diseases. Arch Ophthalmol 105:1660, 1987 106. Adkins JC, Balfour JA: Brimonidine: a review of its pharmacological properties and clinical potential
in the management of open-angle glaucoma and ocular hypertension. Drugs Aging 12:225, 1998 107. Wilson FM: Adverse external ocular effects of topical ophthalmic therapy: an epidemiological, laboratory
and clinical study. Trans Am Ophthalmol Soc 81:854, 1983 108. Curtin BJ, Theodore FH: Ocular molluscum contagiosum. Am J Ophthalmol 39:302, 1955 109. Pettit TH, Holland GM: Chronic keratoconjunctivitis associated with ocular adenoviral infection. Am J Ophthalmol 88:748, 1979 110. Darougar S, Quinlan MP, Gibson JA, et al: Epidemic keratoconjunctivitis and chronic papillary conjunctivitis in London
due to adenovirus type 19. Br J Ophthalmol 61:76, 1977 111. Stenson S: Cytologic diagnosis. In: Laboratory Diagnosis in Ophthalmology. New York, Macmillan, 1987 112. Kobayashi TK, Sato S, Tsubota K, et al: Cytological evaluation of adenoviral follicular conjunctivitis by cytobrush. Ophthalmologica 202:156, 1991 113. Cvenkel B, Globocnik M: Conjunctival scrapings and impression cytology in chronic conjunctivitis. Correlation with microbiology. Eur J Ophthalmol 7:19, 1997 114. Drew WL, Stevens GR: How your laboratory should perform viral studies: isolation and identification
of commonly encountered viruses. Lab Med 11:14, 1980 115. Madhavan HN, Rao SK, Natarajan K, et al: Evaluation of laboratory tests for diagnosis of chlamydial infections in
conjunctival specimens. Indian J Med Res 100:5, 1994 116. Schachter J, Moncada I, Dawson CR, et al: Nonculture methods for diagnosing chlamydial infection in trachoma: a clue
to the pathogenesis of the disease?J Infect Dis 158:1347, 1988 117. Horn JE, Quinn T, Hammer ML, et al: Use of nucleic acid probes for the detection of sexually transmitted agents. Diagn Microbiol Infect Dis 34(suppl):101, 1986 118. Horn JE, Hammer ML, Falkow S, et al: Detection of Chlamydia trachomatis in tissue culture and cervical scrapings by in situ DNA hybridization. J Infect Dis 153:1155, 1986 119. Lee SF, Pepose JS: Sandwich enzyme immunoassay and latex agglutination test for herpes simplex
keratitis. J Clin Microbiol 28:785, 1990 120. Rao NA: A laboratory approach to rapid diagnosis of ocular infections and prospects
for the future. Am J Ophthalmol 107:283, 1989 121. Baveja UK, Hiranandani MK, Talwar P, et al: Laboratory techniques for diagnosis of chlamydial infections of the eye. J Commun Dis 29:247, 1997 122. Numazaki K, Chiba S, Aoki K: Evaluation of serological tests for screening of chlamydial eye diseases. In Vivo 13:235, 1999 123. Wiley L, Springer D, Kowalski RP, et al: Rapid diagnostic test for ocular adenovirus. Ophthalmology 95:431, 1988 124. Bryden AS, Bertrand J: Diagnosis of adenovirus conjunctivitis by enzyme immunoassay. Br J Biomed Sci 53:182, 1996 125. Uchio E, Aoki K, Saitoh W, et al: Rapid diagnosis of adenoviral conjunctivitis on conjunctival swabs by 10-minute
immunochromatography. Ophthalmology 104:1294, 1997 126. Zhang W, Wu Y, Zhao J: Rapid diagnosis and treatment of chlamydial conjunctivitis. Chin Med J (Engl) 108:138, 1995 127. Kowalski RP, Gordon YJ, Romanowski EG, et al: A comparison of enzyme immunoassay and polymerase chain reaction with the
clinical examination for diagnosing ocular herpetic disease. Ophthalmology 100:530, 1993 128. Bersudsky V, Rehany U, Tendler Y, et al: Diagnosis of chlamydial infection by direct enzyme-linked immunoassay and
polymerase chain reaction in patients with acute follicular conjunctivitis. Graefes Arch Clin Exp Ophthalmol 237:617, 1999 129. Talley AR, Garcia-Ferrer F, Laycock KA, et al: Comparative diagnosis of neonatal chlamydial conjunctivitis by polymerase
chain reaction and McCoy cell culture. Am J Ophthalmol 117:50, 1994 130. Talley AR, Garcia-Ferrer F, Laycock KA, et al: The use of polymerase chain reaction for the detection of chlamydial keratoconjunctivitis. Am J Ophthalmol 114:685, 1992 131. Hammerschlag MR, Roblin PM, Gelling M, et al: Use of polymerase chain reaction for the detection of Chlamydia trachomatis in ocular and nasopharyngeal specimens from infants with conjunctivitis. Pediatr Infect Dis J 16:293, 1997 132. Kowalski RP, Uhrin M, Karenchak LM, et al: Evaluation of the polymerase chain reaction test for detecting chlamydial
DNA in adult chlamydial conjunctivitis. Ophthalmology 102:1016, 1995 133. Bailey RL, Hampton TJ, Hayes LJ, et al: Polymerase chain reaction for the detection of ocular chlamydial infection
in trachoma-endemic communities. J Infect Dis 170:709, 1994 134. Elnifro EM, Storey CC, Morris DJ, et al: Polymerase chain reaction for detection of Chlamydia trachomatis in conjunctivalswabs. Br J Ophthalmol 81:497, 1997 135. Morris DJ, Bailey AS, Cooper RJ, et al: Polymerase chain reaction for rapid detection of ocular adenovirus infection. J Med Virol 46:126, 1995 136. Kinchington PR, Turse SE, Kowalski RP, et al: Use of polymerase chain amplification reaction for the detection of adenoviruses
in ocular swab specimens. Invest Ophthalmol Vis Sci 35:4126, 1994 137. Pring-Akerblom P, Trijssenaar FE, Adrian T, et al: Multiplex polymerase chain reaction for subgenus-specific detection of
human adenoviruses in clinical samples. Med Virol 58:87, 1999 138. Dalapathy S, Lily TK, Roy S, et al: Development and use of nested polymerase chain reaction (PCR) for the detection
of adenovirus from conjunctivitis specimens. J Clin Virol 11:77, 1998 139. Takeuchi S, Itoh N, Uchio E, et al: Serotyping of adenoviruses on conjunctival scrapings by PCR and sequence
analysis. J Clin Microbiol 37:1839, 1999 140. Cooper RJ, Yeo AC, Bailey AS, et al: Adenovirus polymerase chain reaction assay for rapid diagnosis of conjunctivitis. Invest Ophthalmol Vis Sci 40:90, 1999 141. Saitoh-Inagawa W, Oshima A, Aoki K, et al: Rapid diagnosis of adenoviral conjunctivitis by PCR and restriction fragment
length polymorphism analysis. J Clin Microbiol 34:2113, 1996 142. Robert PY, Traccard I, Adenis JP, et al: Multiplex detection of herpesviruses in tear fluid using the “stair
primers” PCR method: prospective study of 93 patients. J Med Virol 66:506, 2002 143. Hidalgo F, Melon S, de Ona M, et al: Diagnosis of herpetic keratoconjunctivitis by nested polymerase chain reaction
in human tear film. Eur J Clin Microbiol Infect Dis 17:120, 1998 144. Kowalski RP, Gordon YJ, Romanowski EG, et al: A comparison of enzyme immunoassay and polymerase chain reaction with the
clinical examination for diagnosing ocular herpetic disease. Ophthalmology 100:530, 1993 |