The principal risk factors for yeast or Candida keratitis are protracted epithelial ulceration, penetrating keratoplasty, and therapeutic soft contact lens wear. Concurrent administration of topical corticosteroids enhances the replication of Candida through suppression of the host inflammatory response. Although polymicrobial keratitis is uncommon, risk factors for Candida are comparable to bacterial keratitis, and Candida is the most common genus among mixed bacterial and fungal keratitis.26,27 Polyfungal keratitis is rare.14
The distinct biomicroscopic signs of infectious microbial keratitis are epithelial and stromal ulceration (vs. intact epithelium), necrotizing stromal inflammation (vs. nonnecrotizing inflammation), and single location (vs multifocal sites) of the stromal inflammation. These distinctive signs are determined by the strain (virulence) of the responsible organism(s); the mechanism of inoculation into the cornea; the time interval from inoculation to examination; the antecedent status of the cornea; antimicrobial or corticosteroid therapy; and other host factors, such as an ocular surface abnormality, tear dysfunction state, lid closure dysfunction, and immunosuppression. Other biomicroscopic signs are helpful in judging the severity of the inflammatory process but not the presence or type of infection. These include epithelial edema, stromal edema, loss of endothelial reflectivity (pseudoguttata), endothelial plaque, aqueous flare and cells, and hypopyon.
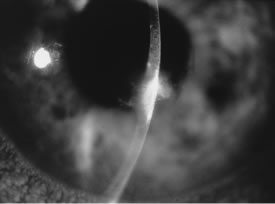
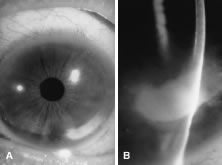
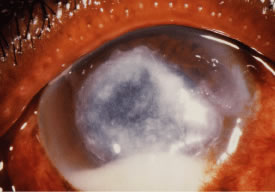
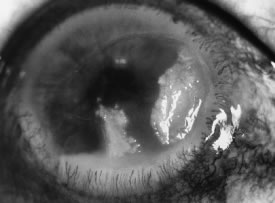
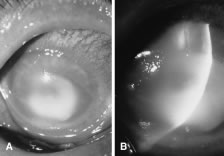
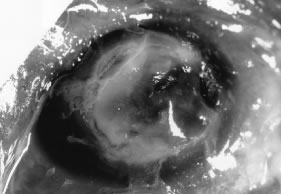
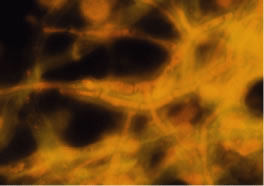
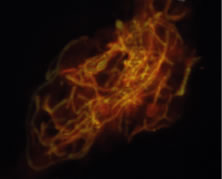
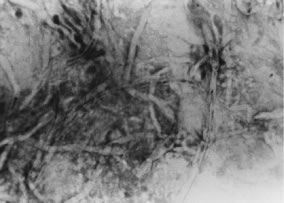
In the early stages of infection, filamentous fungi produce signs that are readily distinguishable from yeast or bacterial keratitis. The most distinctive sign is the presence of delicate, fine, feathery, opalescent, gray-white or yellow-white material in the anterior stroma, surrounded by scant cellular infiltrate or edema (Fig. 1). The epithelium may be intact. The overlying epithelium may be granular and the surface elevated and irregular in contour. Linear infiltrates typically extend into the adjacent stroma. Multiple discrete opacities may develop outside the perimeter of the principal focus of inflammation, either separated by clear stroma or linked by fine linear collections of inflammatory cells and material (Figs. 2 and 3). In the absence of inflammation in the adjacent stroma, branching hyphal fragments may be visualized by biomicroscopy (Figs. 4 and 5). Confocal microscopy may also detect hyphal elements within the stroma.28,29 Peripheral infection resembles noninfectious marginal infiltrative and ulcerative keratitis (Fig. 6). Multifocal keratitis may develop after contact lens wear or injury by multiple projectiles (Fig. 7). In the early stages, iritis is present and the intraocular pressure remains normal. Inappropriate, empirical therapy of fungal keratitis with topical fluoroquinolone or aminoglycoside antibiotics may suppress or eliminate the superficial elements but allow extension of the organisms into the stroma because these agents may possess selective antifungal activity.4,30,31
|
|
|
|
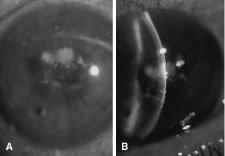
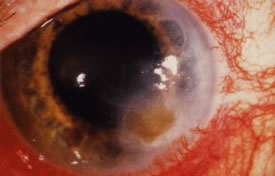
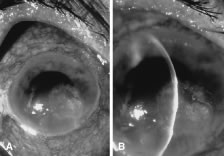
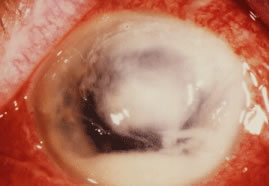
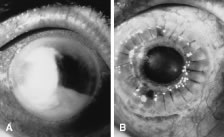
There is no distinguishing clinical sign by which to recognize the genus or species of the infectious filamentous fungus. F. solani is the most virulent organism and typically produces rapidly progressive infection characterized by epithelial and stromal ulceration, dense stromal necrosis, abundant cellular infiltrate, and edema in the adjacent stroma and hypopyon (Figs. 8 and 9). Delicate feathery components are transient. Individual hyphal fragments are rarely visualized. Infection by certain species of Aspergillus and Scedosporium (Figs. 10 and 11) resembles F. solani keratitis and progresses rapidly. Infection by less virulent organisms, such as Curvularia and Alternaria species, produces small, focal (less than 3-mm diameter) areas of nonnecrotizing stromal inflammation with delicate feathery borders (see Fig. 1 and Fig. 12). Macroscopic pigmentation may develop in keratitis caused by Alternaria, Curvularia, and other dematiaceous fungi (Fig. 13).4,11,14 The central component may progress to dense, opaque, gray-white suppuration in the deep stroma without enlargement in total area and may be accompanied by mild inflammation in the adjacent stroma. Iritis is minimal to moderate. Infection caused by other, relatively less virulent organisms resembles herpes simplex or noninfectious keratitis (Fig. 14).
|
|
|
|
|
|
Keratitis caused by Nocardia, nontuberculous Mycobacterium, and other low-virulence bacteria resembles filamentous fungal keratitis by the presence of intact epithelium, multiple stromal infiltrates, and minimal inflammation in the adjacent stroma.
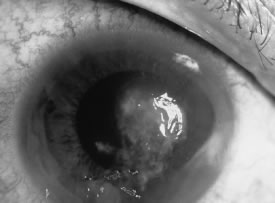
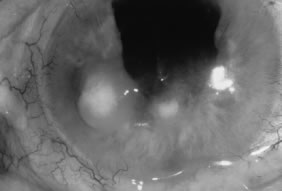
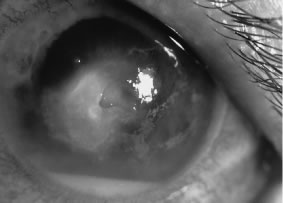
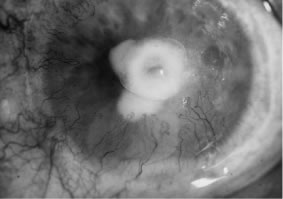
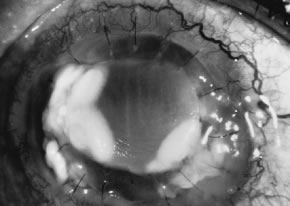
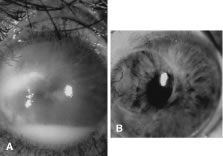
Candida infection typically produces epithelial ulceration, focal necrotizing stromal inflammation, moderate cellular infiltrate and edema in the adjacent stroma, and mild or moderate iritis in the early stages, indistinguishable from bacterial keratitis (Figs. 15, 16, and 17). Fungal elements cannot be detected by biomicroscopy. If untreated, the keratitis evolves to produce dense suppuration and necrosis of the deep stroma. Although multifocal suppuration may develop in polymicrobial keratitis, there is no distinctive sign of mixed Candida and bacterial infection (Fig. 18).
|
|
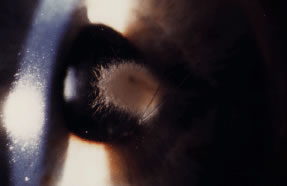
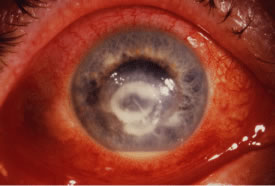
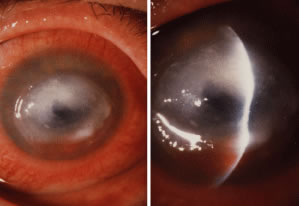
Advanced, severe filamentous fungal or yeast keratitis is indistinguishable from keratitis caused by virulent bacteria such as Staphylococcus aureus or Pseudomonas aeruginosa. The area of epithelial and stromal ulceration is large. Dense, opaque, homogenous, yellow-white stromal necrosis develop and is surrounded by confluent cellular infiltrate and full-thickness stromal edema (Figs. 19, 20, 21). Hyphal elements may penetrate Descemet's membrane and endothelium and be visualized in the anterior chamber. Fibrinous material accumulates over the endothelium, anterior chamber angle, and iris. Pain is typically severe. Secondary ocular hypertension may ensue. Progressive stromal necrosis leads to corneal perforation and, rarely, consecutive endophthalmitis.
|
|