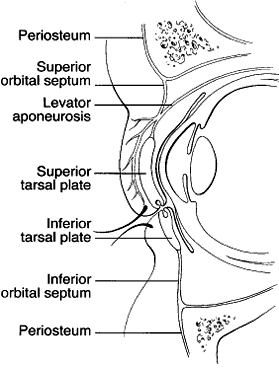
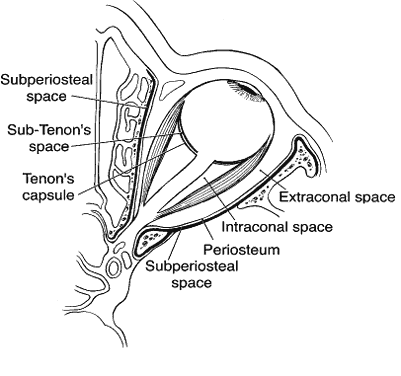
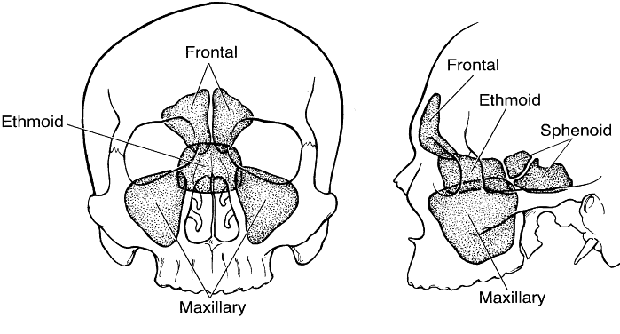
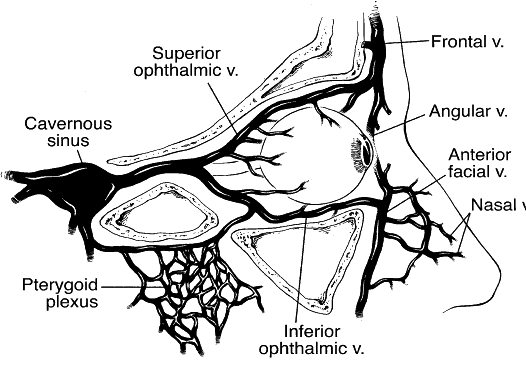
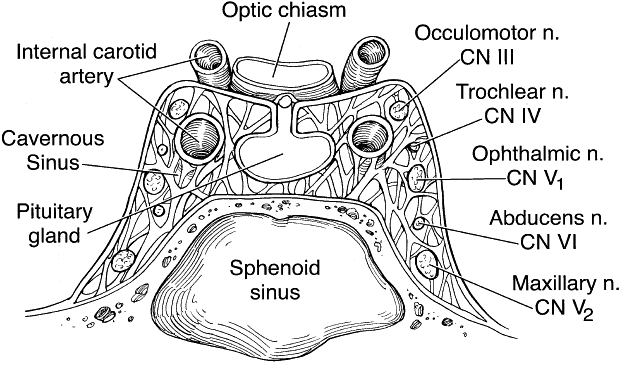
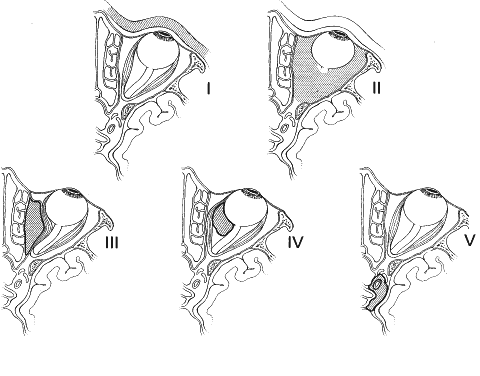
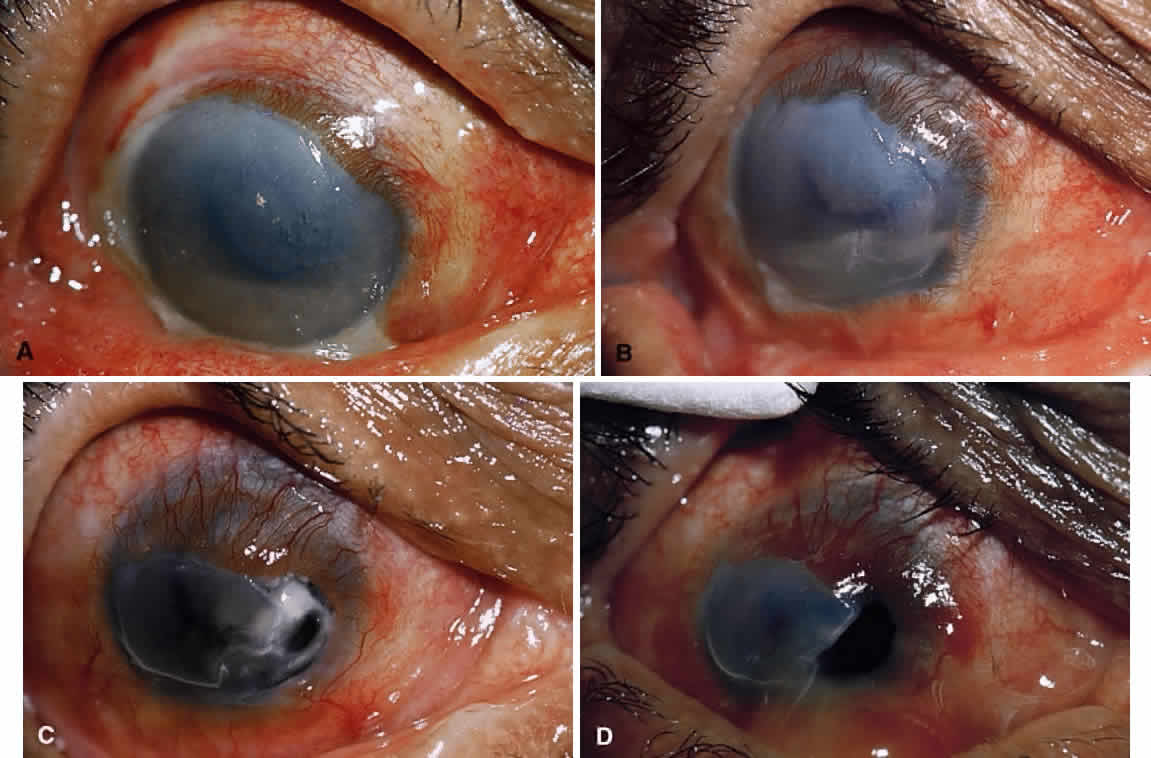
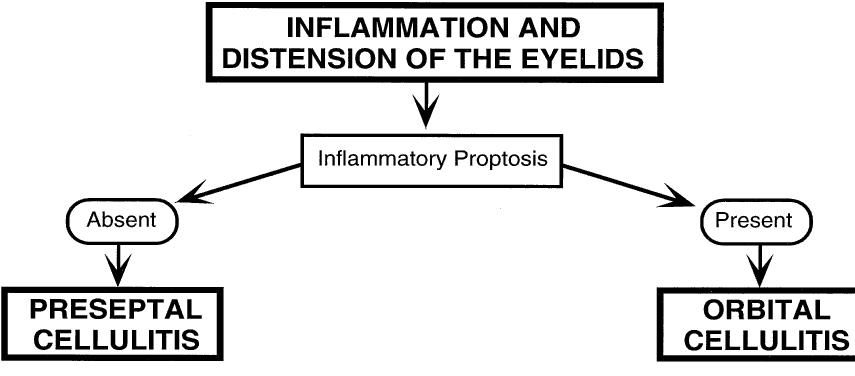
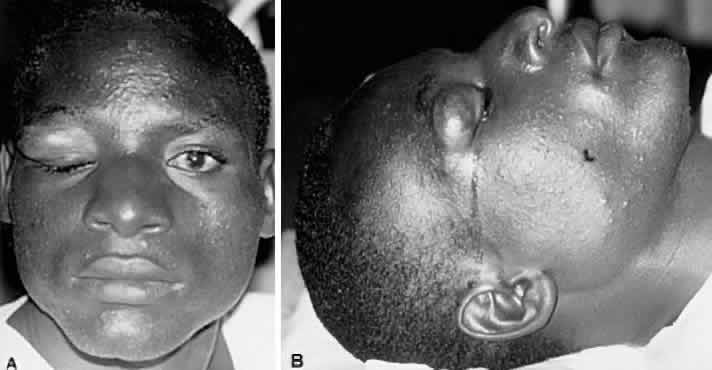
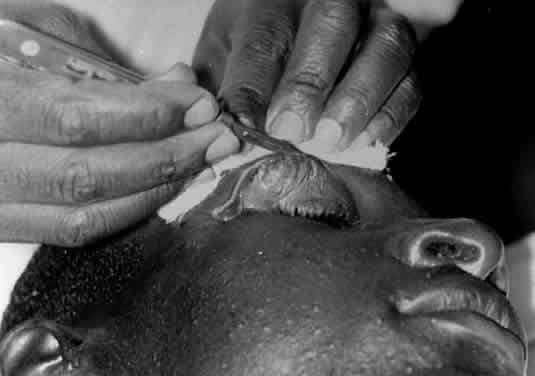
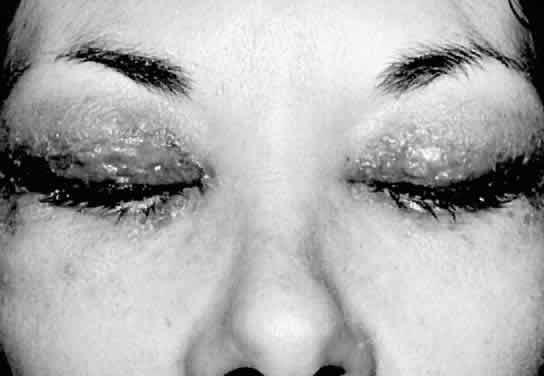
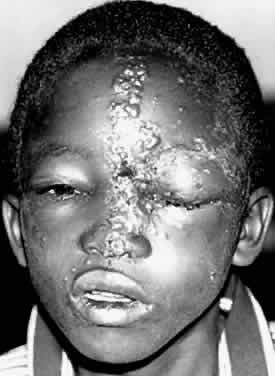
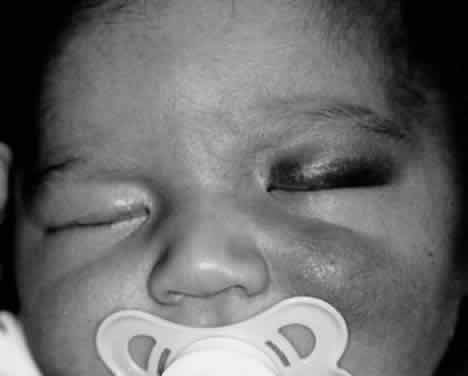
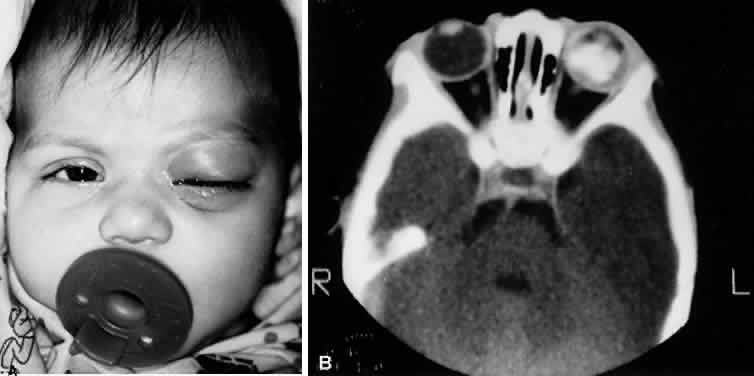
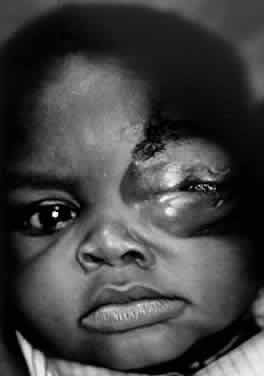
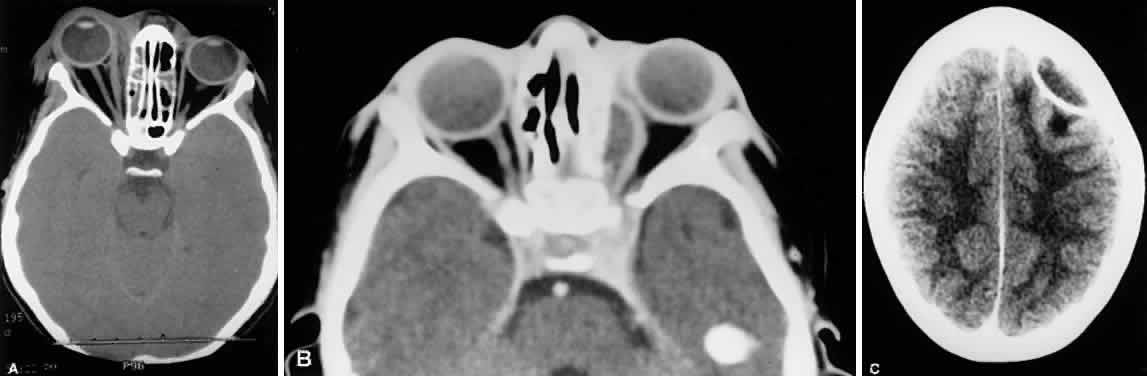
1. Lessner A, Stern GA: Preseptal and orbital cellulitis. Infect Dis Clin North Am 6:933, 1992 2. Koorneef L: Orbital septa: anatomy and function. Ophthalmology 86:876, 1979 3. Doxanas MT, Anderson RL: Clinical Orbital Anatomy. Baltimore, Williams & Wilkins, 1984 4. Maresh MM: Paranasal sinuses from birth to late adolescence. Am J Dis Child 60:55, 1940 5. Hornblass A, Herschorn BJ, Stern K et al: Orbital abscess. Surv Ophthalmol 29:169, 1984 6. Krohel GB, Krauss HR, Winnick J: Orbital abscess. Ophthalmology 89:492, 1982 7. Osborn AG: Craniofacial venous plexuses: angiographic study. Am J Neuroradiol 1:541, 1980 8. Hubert L: Orbital infections due to nasal sinusitis. NY State J Med 37:1559, 1937 9. Smith AT, Spencer JT: Orbital complications resulting from lesions of the sinuses. Ann Otol Rhinol Laryngol 57:5, 1948 10. Chandler JR, Langenbrunner DJ, Stevens ER: The pathogenesis of orbital complications in acute sinusitis. Laryngoscope 80:1414, 1970 11. Spires JR, Smith RJH: Bacterial infections of the orbital and periorbital soft-tissues in children. Laryngoscope 96:763, 1986 12. Weiss A, Friendly D, Eglin K et al: Bacterial periorbital and orbital cellulitis in childhood. Ophthalmology 90:195, 1983 13. Noel LP, Clarke WN, Peacocke TA: Periorbital and orbital cellulitis in childhood. Can J Ophthalmol 16:178, 1981 14. Jackson K, Baker SR: Clinical implications of orbital cellulitis. Laryngoscope 96:568, 1986 15. Eustis HS, Armstrong DC, Buncic JR, Morin JD: Staging of orbital cellulitis in children: computerized tomography characteristics
and treatment guidelines. J Pediatr Ophthalmol Strabismus 23:248, 1986 16. Jones DB, Steinkuller PG: Strategies for the initial management of acute preseptal and orbital cellulitis. Trans Am Ophthalmol Soc 86:94, 1988 17. Jones DB, Matoba AY, Steinkuller PG, Wilhelmus KR: Problem solving in corneal
and external diseases 1996, part I: Adnexa and conjunctiva [course], pp 14–23, 64–74, 80–83. American Academy
of Ophthalmology Annual Meeting, Chicago, 1996 18. Klapper SR, Edmond JC: Initial approach to the child who presents with ocular and periocular infections. Semin Pediatr Infect Dis 7:18, 1996 19. Watters EC, Wallar PH, Hiles DA, Michaels RH: Acute orbital cellulitis. Arch Ophthalmol 94:785, 1976 20. Israele V, Nelson JD: Periorbital and orbital cellulitis. Pediatr Infect Dis J 6:404, 1987 21. Rainey AM, Steinkuller PG: The current etiology of preseptal cellulitis
in children J Pediatr Ophthalmol Strabismus (in press) 22. Adams WG, Deaver KA, Cochi et al: Decline of childhood Haemophilus influenzae type b (Hib) disease in the Hib vaccine era. JAMA 269:221, 1993 23. Redmond SR, Pichicherco ME: Haemophilus influenzae type b disease: an epidemiologic study with special reference to day-care
centers. JAMA 252:2581, 1984 24. Nelson JD, Ginsburg CN: A hypothesis on the pathogenesis of Haemophilus influenzae buccal cellulitis. Pediatrics 88:709, 1976 25. Chartrand SA, Harrison CJ: Buccal cellulitis re-evaluated. Am J Dis Child 140:891, 1986 26. Baker RC, Bausher JC: Meningitis complicating acute bacteremic facial cellulitis. Pediatr Infect Dis 5:421, 1986 27. Noorden CW, Callerame ML, Baum J: Haemophilus influenzae meningitis in an adult: a study of bactericidal antibodies and immunoglobulins. N Engl J Med 282:190, 1970 28. Schneerson R, Rodrigues LP, Parke JC Jr et al:Immunity to disease caused by Haemophilus influenzae type b: II. Specificity and some biologic characteristics of ‘natural,’ infection-acquired, and immunization-induced antibodies
to the capsular polysaccharide of Haemophilus influenzae type b. J Immunol 107:1081, 1971 29. Murphy TF, Apicella MA: Nontypeable Haemophilus influenzae: a review of clinical aspects, surface antigens, and the human immune
response to infection. Rev Infect Dis 9:1, 1987 30. Committee on Infectious Diseases: 1994 Red Book, 23rd ed, pp 203–216, 461. Chicago, American Academy of Pediatrics, 1994 31. Ciarello LR, Rowe PC: Lumbar puncture in children with periorbital and orbital cellulitis. J Pediatr 122:355, 1993 32. Sankrithi UM, Lipuma JJ: Clinically inapparent meningitis complicating acute bacteremic facial cellulitis. Pediatr Emerg Care 7:28, 1991 33. Gellady AM, Shulman ST, Ayoub EM: Periorbital and orbital cellulitis in children. Pediatrics 61:272, 1978 34. Barson WJ, Miller MA, Marcon MJ et al: Cefuroxime therapy for bacteremic soft-tissue infections in children. Am J Dis Child 139:1141, 1985 35. Shapiro ED, Wald ER, Brozarski BA: Periorbital cellulitis and paranasal sinusitis: a reappraisal. Pediatr Infect Dis 1:91, 1982 36. Nelson JD: Cefuroxime: a cephalosporin with unique applicability to pediatric practice. Pediatr Infect Dis 2:394, 1983 37. Gans LA, Shackleford PG: Ocular Infections. In Feigin RD, Cherry JD (eds): Textbook
of Pediatric Infectious Diseases, p 873. Philadelphia, WB
Saunders, 1987 38. Gorbach SL, Bartlett JG: Anaerobic infections. N Engl J Med 290:1177, 1974 39. Placik OJ, Pensler JM, Kim JJ et al: Necrotizing periorbital cellulitis. Ann Plast Surg 31:369, 1993 40. Cox NH, Knowles MA, Porteus ID: Pre-septal cellulitis and facial erysipelas due to Moraxella species. Clin Exp Dermatol 19:321, 1994 41. Lieberman, Brem J: Syndrome of acute osteomyelitis of the superior maxilla in early infancy. N Engl J Med 260:318, 1959 42. Molarte AB, Isenberg SJ: Periorbital cellulitis in infancy. J Pediatr Ophthalmol Strabismus 26:232, 1989 43. Herman J, Katzuni E: Letter to the Editor: Periorbital cellulitis complicating adenovirus infection. Am J Dis Child 140:745, 1986 44. American Academy of Ophthalmology: Basic and Clinical Science Course, Section 7, Orbit, Eyelids, and Lacrimal System: 1995-1996, pp 46–50. San
Francisco, American Academy of Ophthalmology, 1995 45. Allen MV, Cohen KL, Grimson BS: Orbital cellulitis secondary to dacryocystitis following blepharoplasty. Ann Ophthalmol 17:489, 1985 46. Ahrens-Palumbo MJ, Ballen PH: Primary dacryocystitis causing orbital cellulitis. Ann Ophthalmol 14:600, 1982 47. Hemady R, Zaltas M, Paton B et al: Bacillus-induced endophthalmitis: new series of cases and review of the
literature. Br J Ophthalmol 74:26, 1990 48. Bergin DJ, Wright JE: Orbital cellulitis. Br J Ophthalmol 70:174, 1986 49. Shields JA, Shields CL, Suvarnamani C et al: Retinoblastoma manifesting as orbital cellulitis. Am J Ophthalmol 112:442, 1991 50. Grossniklaus HE, Wojno TH: Leukemic infiltrate appearing as periorbital cellulitis. Arch Ophthalmol 108: 484, 1990 51. Clune JP: Septic thrombosis within the cavernous chamber. Am J Ophthalmol 56:33, 1963 52. Harr DL, Quencer RM, Abrams GW: Computerized tomography and ultrasound in the evaluation of orbital infection
and pseudotumor. Neuroradiology 142:395, 1982 53. Lundberg C, Engquist S: Pathogenesis of maxillary sinusitis. Scand J Infect Dis 39(suppl):53, 1983 54. Ekedahl C: Treatment of maxillary sinusitis. Scand J Infect Dis 39(suppl):56, 1983 55. Wald ER: Acute sinusitis and orbital complications in children. Am J Otolaryngol 4:424, 1983 56. Shayegani A, MacFarlane D, Kazim M, Grossman ME: Streptococcal gangrene of the eyelids and orbit. Am J Ophthalmol 120:784, 1995 57. Pillai S, Malone TJ, Abad JC: Orbital tuberculosis. Ophthalmic Plast Reconstr Surg 11:27, 1995 58. Guindi GM: Acute orbital cellulitis: a multidisciplinary emergency. Br J Oral Surg 21:201, 1983 59. Handler LC, Davey IC, Hill JC, Lauryssen C: The acute orbit: differentiation of orbital cellulitis from periosteal
abscess by computerized tomography. Neuroradiology 33: 15, 1991 60. Langham-Brown J, Rhys-Williams S: Computerized tomography of acute orbital infection: the importance of coronal
sections. Clin Radiol 40:471, 1989 61. Lemke BN, Gonnering RS, Harris GJ, Weinstein JM: Orbital cellulitis with periosteal elevation. Ophthalmic Plast Reconstr Surg 3:1, 1987 62. Towbin R, Han BK, Kaufman RA, Burke M: Postseptal cellulitis: CT in diagnosis and management. Radiology 158:735, 1986 63. Schramm VL, Curtin HD, Kennerdell JS: Evaluation of orbital cellulitis and results of treatment. Laryngoscope 92:732, 1982 64. Goodwin WJ, Weinshall M, Chandler JR: The role of high resolution computerized tomography and standardized ultrasound
in the evaluation of orbital cellulitis. Laryngoscope 92:728, 1982 65. Manning SC: Endoscopic management of medial subperiosteal abscess. Arch Otolaryngol Head Neck Surg 119:789, 1993 66. Harris GJ: Subperiosteal inflammation of the orbit: a bacteriological analysis of 17 cases. Arch Ophthalmol 106:947, 1988 67. Catalano RA, Smoot CN: Subperiosteal orbital masses in children with orbital cellulitis: time
for a reevaluation? J Pediatr Ophthalmol Strabismus 27:141, 1990 68. Souliere CR, Antoine GA, Martin MP et al: Selective non-surgical management of subperiosteal abscess of the orbit: computerized
tomography and clinical course as indication for surgical
drainage. Int J Pediatr Otorhinolaryngol 19:109, 1990 69. Rubin SE, Rubin LG, Zito J et al: Medical management of orbital subperiosteal abscess in children. J Pediatr Ophthalmol Strabismus 26:21, 1989 70. Rubin SE, Slavin ML, Rubin LG: Eyelid swelling and erythema as the only signs of subperiosteal abscess. Br J Ophthalmol 73:576, 1989 71. Ferguson EC III: Deep wooden foreign bodies of the orbit: a report of two cases. Trans Am Acad Ophthalmol Otolaryngol 74:778, 1970 72. Green BF, Kraft SP, Carter KD et al: Intraorbital wood: detection by magnetic resonance imaging. Ophthalmology 97:608, 1990 73. Paterson AW, Barnard NA, Irvine GH: Naso-orbital fracture leading to orbital cellulitis, and visual loss as
a complication of chronic sinusitis. Br J Oral Maxillofac Surg 32:80, 1994 74. Silver HS, Fucci MJ, Flanagan JC, Lowry LD: Severe orbital infection as a complication of orbital fracture. Arch Otolaryngol Head Neck Surg 118:845, 1992 75. Westfall CT, Shore JW: Isolated fractures of the orbital floor: risk of infection and the role
of antibiotic prophylaxis. Ophthalmic Surg 22:409, 1991 76. Sevel D, Tobias B, Sellars SL, Forder A: Gas in the orbit associated with orbital cellulitis and paranasal sinusitis. Br J Ophthalmol 57:133, 1973 77. von Noorden GK: Orbital cellulitis following extraocular muscle surgery. Am J Ophthalmol 74:627, 1972 78. Wilson ME, Paul TO: Orbital cellulitis following strabismus surgery. Ophthalmic Surg 18:92, 1987 79. Kivlin JD, Wilson ME Jr: Periocular infection after strabismus surgery: The Periocular Infection
Study Group. J Pediatr Ophthalmol Strabismus 32:42, 1995 80. Jordan DR, Onge PS, Anderson RL et al: Complications associated with alloplastic implants used in orbital fracture
repair. Ophthalmology 89:1600, 1992 81. Mauriello JA, Hargrave S, Yee S et al: Infection after insertion of alloplastic orbital floor implants. Am J Ophthalmol 117:246, 1994 82. Ullman S, Pflugfelder SC, Hughes R, Forster RK: Letter to the Editor: Bacillus cereus panophthalmitis manifesting as an orbital cellulitis. Am J Ophthalmol 105, 1987 83. Harris GJ: Subperiosteal abscess of the orbit. Arch Ophthalmol 101:751, 1983 84. Goto CS, Abramo TJ: Initial approach to the child who presents with oral and dental infections. Semin Pediatr Infect Dis 7(1):55, 1996 85. Gamble RC: Acute inflammations of the orbit in children. Arch Ophthalmol 10:483, 1933 86. Bullock JD, Fleishman JA: Orbital cellulitis following dental extraction. Trans Am Ophthalmol Soc 82:111, 1984 87. Kaban LB, McGill T: Orbital cellulitis of dental origin. J Oral Surg 38:682, 1980 88. Heimtahl A, Nord CE: Orofacial infections of odontogenic origin. Scand J Infect Dis 39(suppl):86, 1983 89. Burnard ED: Proptosis as the first sign of orbital sepsis in the newborn. Br J Ophthalmol 43:9, 1959 90. Hepner R, Hagar D: Staphylococcal orbital sepsis in newborn infants. South Med J 53:922, 1960 91. Patt BS, Manning SC: Blindness resulting from orbital complications of sinusitis. Otolaryngol Head Neck Surg 104:789, 1991 92. Jarrett WH III, Gutman FA: Ocular complications of infections of the paranasal sinuses. Arch Ophthalmol 81:683, 1969 93. Slavin ML, Glaser JS: Acute severe irreversible visual loss with sphenoethmoiditis-‘posterior’ orbital
cellulitis. Arch Ophthalmol 105:345, 1987 94. Ahmadi J, Keane JR, Segall HD, Zee C-S: CT observations pertinent to septic cavernous sinus thrombosis. Am J Neuroradiol 6:755, 1985 95. Fernbach SK, Naidich TP: CT diagnosis of orbital inflammation in children. Neuroradiology 22:7, 1981 96. Lawson W, Blitzer A: Fungal infections of the nose and paranasal sinuses: Part I. Otolaryngol Clin North Am 26: 1007, 1993 97. Maskin SL, Fetchick RJ, Leone CR et al: Bipolaris hawaiiensis—caused phaeohyphomycotic orbitopathy: a devastating
fungal sinusitis in an apparently immunocompetent host. Ophthalmology 96:175, 1989 98. Margo C, Rabinowicz IM, Kwon-Chung KJ, Zimmerman LE: Subacute zygomycosis of the orbit. Arch Ophthalmol 101:1580, 1983 99. Baum JL: Rhino-orbital mucormycosis. Am J Ophthalmol 63:335, 1967 100. Gass JDM: Ocular manifestations of acute mucormycosis. Arch Ophthalmol 65:226, 1961 101. Goodill JJ, Abuelo JG: Letter to the Editor: Mucormycosis: a new risk of deferoxamine therapy
in dialysis patients with aluminum or iron overload? N Engl J Med 317:54, 1987 102. Miller RD, Steinkuller PG, Naegele D: Non-fatal maxillocerebral mucormycosis. Ann Ophthalmol 12:1065, 1980 103. Daly AL, Velazquez LA, Bradley SF, Kauffman CA: Mucormycosis: association with deferoxamine therapy. Am J Med 87:468, 1989 104. Heier JS, Gardner TA, Hawes MJ et al: Proptosis as the initial presentation of fungal sinusitis in immunocompetent
patients. Ophthalmology 102:713, 1995 105. Qingli L, Orcutt JC, Seifter LR: Orbital mucormycosis with retinal and ciliary artery occlusions. Br J Ophthalmol 73:680, 1989 106. Bray WH, Giangiacomo J, Ide CH: Clinical challenges: orbital apex syndrome. Surv Ophthalmol 32:136, 1987 107. Bodenstein NP, McIntosh WA, Vlantis AC, Urquhart AC: Clinical signs of orbital ischemia in rhino-orbitocerebral mucormycosis. Laryngoscope 103:1357, 1993 108. O'Brien TJ, McKelvie P: Rhinocerebral mucormycosis presenting as periorbital cellulitis with blindness: report
of 2 cases. Clin Exp Neurol 31:68, 1994 109. Ferry AP, Abedi S: Diagnosis and management of rhino-cerebral mucormycosis (phycomycosis): a
report of 16 personally observed cases. Ophthalmology 90:1096, 1983 110. Battock DJ, Grausz H, Bobrowski M, Littman ML: Alternate-day amphotericin B therapy in the treatment of rhinocerebral
phycomycosis (mucormycosis). Ann Intern Med 68:122, 1968 111. Marines HM, Osato MS, Font RL: The value of calcofluor white in the diagnosis of mycotic and Acanthamoeba infections of the eye and ocular adnexa. Ophthalmology 94: 23, 1987 112. Couch L, Theilen F, Mader JT: Rhinocerebral mucormycosis with cerebral extension successfully treated
with adjunctive hyperbaric oxygen therapy. Arch Otolaryngol Head Neck Surg 114:791, 1988 113. Stevens MH: Primary fungal infections of the paranasal sinuses. Am J Otolaryngol 2:348, 1981 114. Ferguson BJ, Mitchell TG, Moon R et al: Adjunctive hyperbaric oxygen for treatment of rhinocerebral mucormycosis. Rev Infect Dis 10:551, 1988 115. Galetta SL, Wulc AE, Goldberg HI et al: Rhinocerebral mucormycosis: management and survival after carotid occlusion. Ann Neurol 28:103, 1990 116. Green WR, Font RL, Zimmerman LE: Aspergillosis of the orbit. Arch Ophthalmol 82:302, 1969 117. Rudwan MA, Sheikh HA: Aspergilloma of paranasal sinuses: a common cause of unilateral proptosis
in Sudan. Clin Radiol 27:497, 1976 118. Levin LA, Avery R, Shore JW et al: The spectrum of orbital aspergillosis: a clinicopathological review. Surv Ophthalmol 41:142, 1996 119. Morriss FJ, Spock A: Intracranial aneurysm secondary to mycotic orbital and sinus infection. Am J Dis Child 119: 357, 1970 120. Levine RA: Infection of the orbit by an atypical mycobacterium. Arch Ophthalmol 82:608, 1969 121. Austin P, Dekker A, Kennerdell JS: Orbital aspergillosis: report of a case diagnosed by fine needle aspiration
biopsy. Acta Cytol 27:166, 1983 122. Harris GJ, Will BR: Orbital aspergillosis: conservative debridement and local amphotericin
irrigation. Ophthalmic Plast Reconstr Surg 5:207, 1989 123. Dortzbach RK, Segrest DR: Orbital aspergillosis. Ophthalmic Surg 14:240, 1983 124. Denning DW, Stevens DA: Antifungal and surgical treatment of invasive aspergillosis: a review of 2,121 published
cases. Rev Infect Dis 12:1147, 1990 |