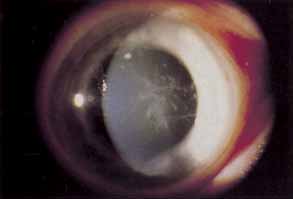
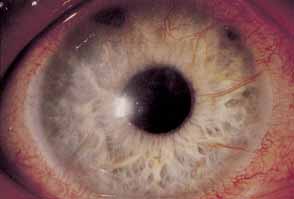
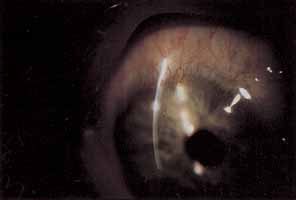
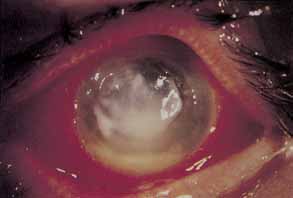
1. Chun M, Weissman BA: Compliance in contact lens care. Am J Optom Physiol Opt 64(4):274–276, 1987 2. Ky W, Scherick K, Stenson S: Clinical survey of lens care in contact lens patients. CLAO J 24(4):216–219, 1998 3. Smith SK: Patient noncompliance with wearing and replacement schedules of disposable
contact lenses. J Am Optom Assoc 67(3):160–164, 1996 4. Dejaco-Ruhswurm I, Scholz U, Hanselmayer G, Skorpik C: Contact lens induced keratitis with contact lens wear. Acta Ophthalmol Scand 79(5):479–483, 2001 5. Zadnik K, Mutti DO, Cutter GR, Chalmers RL: The effect of refractive error on hydrogel contact lens-induced
complications and patient self-management behaviors. Optom Vis Sci 78(9):652–656, 2001 6. Stapleton F, Dart J, Minassian D: Nonulcerative complications of contact lens wear: Relative risks for different
lens types. Arch Ophthalmol 110:1601–1606, 1992 7. Genvert GI, Cohen EJ, Parlato CJ, et al: A prospective study of emergency room visits for contact lens related problems. CLAO J 13(1):42–45, 1987 8. Radford CF, Gastaldo-Brac V, Hill AR: Attendance of contact lens wearers at an ophthalmic accident and emergency
unit. Ophthalmic Physiol Opt 18(1):63–65, 1998 9. Bruce AS, Brennan NA: Corneal pathophysiology with contact lens wear. Surv Ophthalmol 35:25–58, 1990 10. Quinn TG, Schoessler JP: Human corneal epithelial oxygen demand-population characteristics. Am J Optom Physiol Opt 61(6):386–388, 1984 11. Holden BA, Sweeney DF, Sanderson G: The minimum precorneal oxygen tension to avoid corneal edema. Invest Ophthalmol Vis Sci 25:476–480, 1984 12. Polse KA: Factors controlling oxygen tension under a hydrogel contact lens. J Am Optom Assoc 52(3):203–208, 1981 13. Stefansson E, Foulks GN, Hamilton RC: The effect of corneal contact lenses on the oxygen tension in the anterior
chamber of the rabbit eye. Invest Ophthalmol Vis Sci 28:1716–1719, 1987 14. McNamara NA, Polse KA, Brand RJ, et al: Tear mixing under a soft contact lens: Effect of lens diameter. Am J Ophthalmol 127(6):659–665, 1999 15. Stefansson E, Wolbarsht ML, Landers MB: The corneal contact lens and aqueous humor hypoxia in cats. Invest Ophthalmol Vis Sci 24:1052–1054, 1983 16. Giasson C, Bonanno JA: Corneal epithelial and aqueous humor acidification during in vivo contact
lens wear in rabbits. Invest Ophthalmol Vis Sci 35(3):851–861, 1994 17. Harvitt DM, Bonanno JA: PH dependence of corneal oxygen consumption. Invest Ophthalmol Vis Sci 39(13):2778–2781, 1998 18. Kumar P, Elston R, Black D, et al: Allergic rhinoconjunctivitis and contact lens intolerance. CLAO J 17(1):31–34, 1991 19. Ehlers WH, Donshik PC: Allergic ocular disorders: A spectrum of diseases. CLAO J 18(2):117–124, 1992 20. Dart JKG: Disease and risks associated with contact lenses. Br J Ophthalmol 77:49–53, 1993 21. Hardman Lea SJ, Neugebauer MAZ, Smith RG, Vernon SA: The incidence of ophthalmic problems in the contact lens wearing population. Eye 4:706–711, 1990 22. Nilsson SE: Bacterial keratitis and inflammatory corneal reactions: Possible relations
to contact lens oxygen transmissibility: the Harold A. Stein Lectureship 2001. CLAO J 28(2):62–65, 2002 23. Dumbleton K: Noninflammatory silicone hydrogel contact lens complications. CLAO J 29(1 suppl):S186–S189, 2003 24. Lee KY, Lim L: Pseudomonas keratitis associated with continuous wear silicone-hydrogel
soft contact lens: A case report. CLAO J 29(4):255–257, 2003 25. Yeniad B, Adam YS, Bilgin LK, Gozum N: Effect of 30-day continuous wear of silicone hydrogel lenses on
corneal thickness. CLAO J 30(1):6–9, 2004 26. Rapkin JS: The effect of daily wear time on contact lens complications. CLAO J 14(3):139–142, 1988 27. Keech PM, Ichikawa L, Barlow W: A prospective study of contact lens complications in a managed care setting. Optom Vis Sci 73(10):653–658, 1996 28. Levy B, McNamara N, Corzine J, Abbott RL: Prospective trial of daily and extended wear disposable contact lenses. Cornea 16(3):274–276, 1997 29. Hamano H, Watanabe K, Mamano T, et al: A study of the complications induced by conventional and disposable contact
lenses. CLAO J 20(2):103–108, 1994 30. Pritchard N, Fonn D, Weed K: Ocular and subjective responses to frequent replacement of daily wear soft
contact lenses. CLAO J 22(1):53–59, 1996 31. Suchecki JK, Ehlers WH, Donshik PC: A comparison of contact lens-related complications in various daily
wear modalities. CLAO J 26(4):204–213, 2000 32. Ilhan B, Irkec M, Orhan M, Celik H: Surface deposits on frequent replacement and conventional daily wear soft
contact lenses: A scanning electron microscopic study. CLAO J 24(4):232–235, 1998 33. Solomon OD, Freman MI, Boshnik EL, et al: A 3-year prospective study of the clinical performance of daily
disposable contact lenses compared with frequent replacement and conventional
daily wear contact lenses. CLAO J 22(4):250–257, 1996 34. Bennett ES, Egan DJ: Rigid gas-permeable lens problem solving. J Am Optom Assoc 57(7):504–511, 1986 35. Fowler SA, Korb DR, Finnemore VM, Allansmith MR: Surface deposits on worn hard contact lenses. Arch Ophthalmol 102:757–759, 1984 36. Tripathi RC, Tripathi BJ, Silverman RA, Rao GN: Contact lens deposits and spoilage: Identification and management. Int Ophthalmol Clin 31(2):91–120, 1991 37. Kenyon E, Polse KA, Mandell RB: Rigid contact lens adherence: Incidence, severity and recovery. J Am Optom Assoc 59(3):168–174, 1988 38. Swarbrick HA, Holden BA: Rigid gas permeable lens binding: Significance and contributing factors. Am J Optom Physiol Opt 64(11):815–823, 1987 39. Poggio EC, Abelson M: Complications and symptoms with disposable daily wear contact lenses and
conventional soft daily wear contact lenses. CLAO J 19(2):95–102, 1993 40. Poggio EC, Abelson M: Complications and symptoms in disposable extended wear lenses compared
with conventional soft daily wear lenses and soft extended wear lenses. CLAO J 19(1):31–39, 1993 41. Lamer L: Extended wear contact lenses for myopes: A follow-up study of 400 cases. Ophthalmology 90:156–161, 1983 42. Weissman BA, Pearson TR: Clinical management of cosmetic extended-wear contact lens failure. J Am Optom Assoc 57(6):448–450, 1986 43. Holden BA, Swarbrick HA, Sweeney DF, et al: Strategies for minimizing the ocular effects of extended contact lens wear: A
statistical analysis. Am J Optom Physiol Opt 64(10):781–789, 1987 44. Taylor DSI: Risks and difficulties of the treatment of aphakia in infancy. Trans Ophthalmol Soc UK 102:403–406, 1982 45. Graham CM, Dart JKG, Buckley RJ: Extended wear hydrogel and daily wear hard contact lenses for aphakia: Success
and complications compared in a longitudinal study. Ophthalmology 93:1489–1494, 1986 46. Spoor TC, Hartel WC, Wynn P, Spoor CK: Complications of continuous-wear soft contact lenses in a nonreferral
population. Arch Ophthalmol 102:1312–1313, 1984 47. Hayworth NAS, Asbell PA: Therapeutic contact lenses. CLAO J 16(2):137–142, 1990 48. McDermott ML, Chandler JW: Therapeutic uses of contact lenses. Surv Ophthalmol 33:381–394, 1989 49. Ong BL, Larke JR: Meibomian gland dysfunction: Some clinical, biochemical and physical observations. Ophthalmic Physiol Opt 10(2):144–148, 1990 50. van den Bosch WA, Lemji HG: Blepharoptosis induced by prolonged hard contact lens wear. Ophthalmology 99:1759–1765, 1992 51. Epstein G, Putterman A: Acquired blepharoptosis secondary to contact-lens wear. Am J Ophthalmol 91:634–639, 1981 52. Bellan L, Buffam F: The ‘O’ sign-clue to a lost lens. Can J Ophthalmol 25(7):348–350, 1990 53. Jones D, Livesy S, Wilkins P: Hard contact lens migration into the upper lid: An unexpected lump. Br J Ophthalmol 71:368–370, 1987 54. Sebag J, Albert DM: Pseudochalazion of the upper lid due to hard contact lens embedding-case
reports and literature review. Ophthalmic Surg 13(8):634–636, 1982 55. Brinkley JR, Zappia RJ: An eyelid tumor caused by a migrated hard contact lens. Ophthalmic Surg 11(3):200–202, 1980 56. Friedberg ML, Abedi S: Encysted hard contact lens appearing as an orbital mass. Ophthal Plast Reconstr Surg 5(4):291–293, 1989 57. Roberts-Harry TJ, Cavey CC, Jagger JD: Periocular migration of hard contact lenses. Br J Ophthalmol 76(2):95–97, 1992 58. Korb DR, Allansmith MR, Greiner JV, et al: Prevalence of conjunctival changes in wearers of hard contact lenses. Am J Ophthalmol 90:336–341, 1980 59. Greiner JV, Allansmith MR: Effect of contact lens wear on the conjunctival mucous system. Ophthalmology 88:821–832, 1981 60. Gilbard JP, Gray KL, Rossi SR: A proposed mechanism for increased tear-film osmolarity in contact
lens wearers. Am J Ophthalmol 102:505–507, 1986 61. Farris RL: The dry eye: Its mechanisms and therapy, with evidence that contact lens
is a cause. CLAO J 12(4):234–246, 1986 62. Reitschel RL, Wilson LA: Ocular inflammation in patients using soft contact lenses. Arch Dermatol 118:147–149, 1982 63. Allansmith MR, Ross RN: Giant papillary conjunctivitis. Int Ophthalmol Clin 28(4):309–316, 1988 64. Donshik PC: Giant papillary conjunctivitis. Trans Am Ophthalmol Soc 92:687–744, 1994 65. Douglas JP, Lowder CY, Lazorik R, Meisler DM: Giant papillary conjunctivitis associated with rigid gas-permeable
contact lenses. CLAO J 14(3):143–147, 1988 66. Begley CG, Riggle A, Tuel JA: Association of giant papillary conjunctivitis with seasonal allergies. Optom Vis Sci 67(3):192–195, 1990 67. Allansmith MR, Baird RS: Percentage of degranulated mast cells in vernal conjunctivitis and giant
papillary conjunctivitis associated with contact-lens wear. Am J Ophthalmol 91:71–75, 1981 68. Barishak Y, Zavaro A, Samra Z, Sompolinsky D: An immunological study of papillary conjunctivitis due to contact lenses. Curr Eye Res 3(10):1161–1168, 1984 69. Goen TM, Sieboldt K, Terry JE: Cromolyn sodium in ocular allergic diseases. J Am Optom Assoc 57(7):526–529, 1986 70. Kruger CJ, Ehlers WH, Luistro AE, Donshik PC: Treatment of giant papillary conjunctivitis with cromolyn sodium. CLAO J 18(1):46–48, 1992 71. Wood TS, Stewart RH, Bowman RW, et al: Suprofen treatment of contact lens-associated giant papillary conjunctivitis. Ophthalmology 95:822–826, 1988 72. Bucci FA, Lopatynsky MO, Jenkins PL, et al: Comparison of the clinical performance of the Acuvue disposable contact
lens and the CSI lens in patients with giant papillary conjunctivitis. Am J Ophthalmol 115:454–459, 1993 73. Porazinski AD, Donshik PC: Giant papillary conjunctivitis in frequent replacement contact lens wearers: A
retrospective study. CLAO J 25(3):142–147, 1999 74. Sendele DD, Kenyon KR, Mobilia EF, et al: Superior limbic keratoconjunctivitis in contact lens wearers. Ophthalmology 90:616–622, 1983 75. Fuerst DJ, Sugar J, Worobec S: Superior limbic keratoconjunctivitis associated with cosmetic soft contact
lens wear. Arch Ophthalmol 101:1214–1216, 1983 76. Hamburger HA: Pyogenic granuloma associated with extended wear contact lenses. CLAO J 12(2):99–100, 1986 77. Horton JC, Mathers WD, Zimmerman LE: Pyogenic granuloma of the palpebral conjunctiva associated with contact
lens wear. Cornea 9(4):359–361, 1990 78. Ichijima H, Petroll WM, Jester JV, et al: Effects of increasing dk with rigid contact lens extended wear on rabbit
corneal epithelium using confocal microscopy. Cornea 11(4):282–287, 1992 79. Lemp MA, Gold JB: The effects of extended-wear hydrophilic contact lenses on the corneal
epithelium. Am J Ophthalmol 101:274–277, 1986 80. Tripathi BJ, Tripathi RC: Hydrogen peroxide damage to human corneal epithelial cells in vitro: Implications
for contact lens disinfection systems. Arch Ophthalmol 107:1516–1519, 1989 81. Clinch TE, Goins KM, Cobo LM: Treatment of contact lens-related ocular surface disorder with autologous
conjunctival transplantation. Ophthalmology 99:634–638, 1992 82. Guex-Crosier Y, Herbot CP: Presumed corneal intraepithelial neoplasia associated with contact lens
wear and intense ultraviolet light exposure. Br J Ophthalmol 77:191–192, 1993 83. Bergmanson JPG, Chu LWF: Contact lens-induced corneal epithelial injury. Am J Optom Physiol Opt 59(6):500–506, 1982 84. Bloomfield SE, Jakobiec FA, Theodore FH: Contact lens induced keratopathy: A severe complication extending the spectrum
of keratoconjunctivitis in contact lens wearers. Ophthalmology 91:290–294, 1984 85. Lohman LE: Corneal epithelial response to contact lens wear. CLAOJ 12(3):153–156, 1986 86. Udell IJ, Mannis MJ, Meisler DM, Langston RHS: Pseudodendrites in soft contact lens wearers. CLAO J 11(1):51–53, 1985 87. Margulies LJ, Mannis MJ: Dendritic corneal lesions associated with soft contact lens wear. Arch Ophthalmol 101:1551–1553, 1983 88. Seedor JA, Waring GO: Dendriform lesions of the cornea induced by soft contact lenses. Arch Ophthalmol 105:1021, 1987 89. Stainer GA, Brightbill FS, Holm P, Laux D: The development of pseudopterygia in hard contact lens wearers. Contact and Intraocul Lens Med J 7(1):1–4, 1981 90. Hayasaka S, Morihiro K, Shibasaki H, et al: Superficial punctate keratopathy and bacterial growth in patients with
unilateral aphakia using extended-wear soft contact lenses. Ophthalmologica 204:169–174, 1992 91. Rosenfeld SI, Mandelbaum S, Corrent GF, et al: Granular epithelial keratopathy as an unusual manifestation of pseudomonas
keratitis associated with extended-wear soft contact lenses. Am J Ophthalmol 109:17–22, 1990 92. Lindquist TD, Sher NA, Doughman DJ: Clinical signs and medical therapy of early acanthamoeba keratitis. Arch Ophthalmol 106:73–77, 1988 93. Madigan MC, Holden BA, Kwok LS: Extended wear of contact lenses can compromise corneal epithelial adhesion. Curr Eye Res 6(10):1257–1260, 1987 94. McMonnies CW: Contact lens intolerance in association with epithelial dystrophy. Am J Optom Physiol Opt 58(5):414–418, 1981 95. Udell IJ, Ormerod LD, Boniuk V, Abelson MB: Treatment of contact lens-associated corneal erosions (Letter). Am J Ophthalmol 104:306–307, 1987 96. Dangel ME, Kracher GP, Stark WJ: Anterior corneal mosaic in eyes with keratoconus wearing hard contact lenses. Arch Ophthalmol 102:888–890, 1984 97. Mobilia EF, Yamamoto GK, Dohlman CH: Corneal wrinkling induced by ultra-thin soft contact lenses. Ann Ophthalmol 103:371–375, 1980 98. Humphreys JA, Larke JR, Parrish ST: Microepithelial cysts observed inextended contact-lens wearing subjects. Br J Ophthalmol 64:888–889, 1980 99. Holden BA, Sweeney DF: The significance of the microcyst response: A review. Optom Vis Sci 68(9):703–707, 1991 100. Kenyon E, Polse KA, Seger RG: Influence of wearing schedule on extended-wear complications. Ophthalmology 93:231–236, 1986 101. Arentsen JJ: Corneal neovascularization in contact lens wearers. Int Ophthalmol Clin 26(1):15–23, 1986 102. Efron N: Vascular response of the cornea to contact lens wear. J Am Optom Assoc 58(10):836, 1987 103. McMonnies CW, Chapman-Davies A, Holden BA: The vascular response to contact lens wear. Am J Optom Physiol Opt 59(10):795–799, 1982 104. Duffin RM, Weissman BA, Glasser DB, Pettit TH: Flurbiprofen in the treatment of corneal neovascularization induced by
contact lenses. Am J Ophthalmol 93:607–614, 1982 846, 1987 105. Lemp MA: The effect of extended-wear aphakic hydrophilic contact lenses after
penetrating keratoplasty. Am J Ophthalmol 90:331–335, 1980 106. Bergenske PD, Polse KA: The effect of rigid gas permeable lenses on corneal sensitivity. J Am Optom Assoc 58(3):212–215, 1987 107. Beuerman RW, Rozsa AJ: Threshold and signal detection measurements of the effect of soft contact
lenses on corneal sensitivity. Curr Eye Res 4(6):742–744, 1985 108. Rohde MD, Huff JW: Contact lens-induced edema in vitro-amelioration by lactate
dehydrogenase inhibitors. Curr Eye Res 5(10):751–758, 1986 109. Bergmanson JPG, Chu LWF: Corneal response to rigid contact lens wear. Br J Ophthalmol 66:667–675, 1982 110. Johnson MH, Ruben CM, Perrigin DM: Entoptic phenomena and reproducibility of corneal striae following contact
lens wear. Br J Ophthalmol 71:737–741, 1987 111. Lambert SR, Klyce SD: The origins of Sattler's veil. Am J Ophthalmol 91:51–56, 1981 112. Nirankari VS, Baer JC: Persistent corneal edema in aphakic eyes from daily-wear and extended-wear
contact lenses. Am J Ophthalmol 98:329–335, 1984 113. Holden BA, Mertz GW: Critical oxygen levels to avoid corneal edema for daily and extended wear
contact lenses. Invest Ophthalmol Vis Sci 25:1161–1167, 1984 114. O'Neal MR, Polse KA, Sarver MD: Corneal response to rigid and hydrogel lenses during eye closure. Invest Ophthalmol Vis Sci 25:837–842, 1984 115. Sarver MD, Polse KA, Baggett DA: Intersubject difference in corneal edema response to hypoxia. Am J Optom Physiol Opt 60(2):128–131, 1983 116. Holden BA, Mertz GW, McNally JJ: Corneal swelling response to contact lenses worn under extended wear conditions. Invest Ophthalmol Vis Sci 24:218–226, 1983 117. Sarver MD, Baggett DA, Harris MG, Louie K: Corneal edema with hydrogel lenses and eye closure: Effects of oxygen transmissibility. Am J Optom Physiol Opt 58(5):386–392, 1981 118. Nishida T, Yasumoto K, Morikawa Y, Otori T: Hard contact lens-induced corneal neovascularization treated by
oxygenation. Cornea 10(4):358–360, 1991 119. Rozenman Y, Donnenfeld ED, Cohen EJ, et al: Contact lens-related deep stromal neovascularization. Am J Ophthalmol 107:27–32, 1989 120. Nirankari VS, Karesh J, Lakhanpal V, Richards RD: Deep stromal vascularization associated with cosmetic, daily-wear
contact lenses. Arch Ophthalmol 101:46–47, 1983 121. Al-Hussaini AK, Friedlander MH, Karcioglu ZA: Intracorneal hemorrhage secondary to aphakic contact lens wear. Cornea 11(1):73–76, 1992 122. Donnenfeld ED, Ingraham H, Perry HD, et al: Contact lens-related deep stromal intracorneal hemorrhage. Ophthalmology 98:1793–1796, 1991 123. Yeoh RLS, Cox N, Falcon MG: Spontaneous intracorneal haemorrhage. Br J Ophthalmol 73:363–364, 1989 124. Braude LS, Sugar J: Circinate-pattern interstitial keratopathy in daily wear soft contact
lens wearers. Arch Ophthalmol 103:1662–1665, 1985 125. Conway ST, Wagdi SF: Corneal scarring associated with daily soft contact lens wear. Ann Ophthalmol 15(9):868–871, 1983 126. Horowitz GS, Lin J, Chew HC: An unusual corneal complication of soft contact lens. Am J Ophthalmol 100:794–797, 1985 127. Chayet AS, Chayet J: An unusual corneal complication of soft contact lens (Letter). Am J Ophthalmol 101:623, 1986 128. Korb DR, Finnemore VM, Herman JP: Apical changes and scarring in keratoconus as related to contact lens fitting
techniques. J Am Optom Assoc 53(3):199–205, 1982 129. Remeijer H, van Rij G, Beekhuis WH, et al: Deep corneal stromal opacities in long-term contact lens wear. Ophthalmology 97:281–285, 1990 130. Aquavella JV, DePaolis MD: Sterile infiltrates associated with contact lens wear. Int Ophthalmol Clin 31(2):121–126, 1991 131. Stein RM, Clinch TE, Cohen EJ, et al: Infected vs sterile corneal infiltrates in contact lens wearers. Am J Ophthalmol 105:632–636, 1988 132. Suchecki JK, Ehlers WH, Donshik PC: Peripheral corneal infiltrates associated with contact lens wear. CLAO J 22(1):41–46, 1996 133. Gordon A, Kracher GP: Corneal infiltrates and extended-wear contact lenses. J Am Optom Assoc 56(3):198–201, 1985 134. Bates AK, Morris RJ, Stapleton F, et al: Sterile corneal infiltrates in contact lens wearers. Eye 3:803–810, 1989 135. Crook T: Corneal infiltrates with red eye related to duration of extended wear. J Am Optom Assoc 56(9):698–700, 1985 136. Maguen E, Rosner I, Caroline P, et al: A retrospective study of disposable extended wear lenses in 100 patients: Year 2. CLAO J 18(4):229–231, 1992 137. Mondino BJ, Groden LR: Conjunctival hyperemia and corneal infiltrates with chemically disinfected
soft contact lenses. Arch Ophthalmol 98:1167–1170, 1980 138. Stern GA: Corneal infections in contact lens wearers. Int Ophthalmol Clin 31(2):147–161, 1991 139. Poggio EC, Glynn RJ, Schein OD, et al: The incidence of ulcerative keratitis among users of daily-wear
and extended-wear soft contact lenses. N Engl J Med 321:779–783, 1989 140. Schein OD, Glynn RJ, Poggio EC, et al: Microbial Keratitis Study Group: The relative risk of ulcerative keratitis
among users of daily-wear and extended-wear soft contact
lenses. N Engl J Med 321:773–778, 1989 141. Glynn RJ, Schein OD, Seddon JM, et al: The incidence of ulcerative keratitis among aphakic contact lens wearers
in New England. Arch Ophthalmol 109:104–107, 1991 142. Galentine PG, Cohen EJ, Laibson PR, et al: Corneal ulcers associated with contact lens wear. Arch Ophthalmol 102:891–894, 1984 1986 143. Alfonso E, Mandelbaum S, Fox MJ, Forster RK: Ulcerative keratitis associated with contact lens wear. Am J Ophthalmol 101:429–433, 1986 144. Mondino BJ, Weissman BA, Farb MD, Pettit TH: Corneal ulcers associated with daily-wear and extended-wear
contact lenses. Am J Ophthalmol 102:58–65, 1986 145. Ormerod LD, Smith RE: Contact lens-associated microbial keratitis. Arch Ophthalmol 104:79–83, 1986 146. Chalupa E, Swarbrick HA, Holden BA, Sjostrand J: Severe corneal infections associated with contact lens wear. Ophthalmology 94:17–22, 1987 147. Wilhelmus KR: Review of clinical experience with microbial keratitis associated with
contact lenses. CLAO J 13(4):211–214, 1987 148. Derick RJ, Kelley CG, Gersman M: Contact lens related corneal ulcers at the Ohio State University Hospitals 1983–1987. CLAO J 15(4):268–270, 1989 149. Koidou-Tsiligianni A, Alfonso E, Forster RK: Ulcerative keratitis associated with contact lens wear. Am J Ophthalmol 108:64–67, 1989 150. Schein OD, Poggio EC: Ulcerative keratitis in contact lens wearers. Cornea 9(suppl 1):S55–S58, 1990 151. Cohen EJ, Gonzalez C, Leavitt KG, et al: Corneal ulcers associated with contact lenses including experience with
disposable lenses. CLAO J 17(3):173–176, 1991 152. MacRae S, Herman C, Stulting D, et al: Corneal ulcer and adverse reaction rates in premarket contact lens studies. Am J Ophthalmol 111:457–465, 1991 153. Donnenfeld ED, Cohen EJ, Arentsen JJ, et al: Changing trends in contact lens associated corneal ulcers: An overview
of 116 cases. CLAO J 12(3):145–149, 1986 154. Schein OD, Ormerod LD, Barraquer E, et al: Microbiology of contact lens-related keratitis. Cornea 8(4):281–285, 1989 155. Dart JKG: Predisposing factors in microbial keratitis: The significance of contact
lens wear. Br J Ophthalmol 72:926–930, 1988 156. Musch DC, Sugar A, Meyer RF: Demographic and predisposing factors in corneal ulceration. Arch Ophthalmol 101:1545–1548, 1983 157. Rabinovitch J, Cohen EJ, Genvert GI, et al: Seasonal variation in contact lens-associated corneal ulcers. Can J Ophthalmol 22(3):155–156, 1987 158. Franks WA, Adams GGW, Dart JKG, Minassian D: Relative risks of different types of contact lenses. Br Med J 297:520–521, 1988 159. Aswad MI, John T, Barza M, et al: Bacterial adherence to extended wear soft contact lenses. Ophthalmol 97:296–302, 1990 160. Kent HD, Cohen EJ, Laibson PR, Arentsen JJ: Microbial keratitis and corneal ulceration associated with therapeutic
soft contact lenses. CLAO J 16(1):49–52, 1990 161. Leibowitz HM: Clinical evaluation of ciprofloxacin 0.3% ophthalmic solution for
treatment of bacterial keratitis. Am J Ophthalmol 112:34S–47S, 1991 162. Bowden FW, Cohen EJ, Arentsen JJ, Laibson PR: Patterns of lens care practices and lens product contamination in contact
lens associated microbial keratitis. CLAO J 15(1):49–54, 1989 163. Wilson LA, Sawant AD, Simmons RB, Ahearn DG: Microbial contamination of contact lens storage cases and solutions. Am J Ophthalmol 110:193–198, 1990 164. Bilgin LK, Manav G, Tutkun IT, et al: Efficacy of a one-step hydrogen peroxide system for disinfection
of soft contact lenses. CLAO J 19(1):50–52, 1993 165. Stern GA, Zam ZS: The effect of enzymatic contact lens cleaning on the adherence of pseudomonas aeruginosa to soft contact lenses. Ophthalmology 95:115–119, 1987 166. Adams CP, Cohen EJ, Laibson PR, et al: Corneal ulcers in patients with cosmetic extended-wear contact lenses. Am J Ophthalmol 96:705–709, 1983 167. Cohen EJ Laibson PJ, Arentsen JJ, Clemons CS: Corneal ulcers associated with cosmetic extended wear soft contact lenses. Ophthalmology 94:109–114, 1987 168. Weissman BA, Mondino BJ, Pettit TH, Hofbauer JD: Corneal ulcers asociated with extended-wear soft contact lenses. Am J Ophthalmol 97:476–481, 1984 169. Eichenbaum JW, Feldstein M, Podos SM: Extended-wear aphakic soft contact lenses and corneal ulcers. Br J Ophthalmol 66:663–666, 1982 170. Lemp MA, Blackman HJ, Wilson LA, Leveille AS: Gram-negative corneal ulcers in elderly aphakic eyes with extended
wear lenses. Ophthalmol 90:60–63, 1984 171. Klotz SA, Misra RP, Butrus SI: Contact lens wear enhances adherence of pseudomonas aeruginosa and binding of lectins to the cornea. Cornea 9(3):266–270, 1990 172. Boles SF, Refojo MF, Leong F: Attachment of pseudomonas to human-worn, disposable etafilcon A contact lenses. Cornea 11(1):47–52, 1992 173. Beuhler PO, Schein OD, Stamler JF, et al: The increased risk of ulcerative keratitis among disposable soft contact
lens users. Arch Ophthalmol 110:1555–1558, 1992 174. Dunn JP, Mondino BJ, Weissman BA, et al: Corneal ulcers associated with disposable hydrogel contact lenses. Am J Ophthalmol 108:113–117, 1989 175. Wilhelmus KR, Robinson NM, Font RA, et al: Fungal keratitis in contact lens wearers. Am J Ophthalmol 106:708–714, 1988 176. Wilson LA, Ahearn DG: Association of fungi with extended-wear soft contact lenses. Am J Ophthalmol 101:434–436, 1986 177. Berger RO, Streeten BW: Fungal growth in aphakic soft contact lenses. Am J Ophthalmol 91:630–633, 1981 178. Churner R, Cunningham RD: Fungal-contaminated soft contact lenses. Ann Ophthalmol 15(8):724–727, 1983 179. Moore MB, McCulley JP, Luckenbach M, et al: Acanthamoeba keratitis associated with soft contact lenses. Am J Ophthalmol 100:396–403, 1985 180. Stehr-Green JK, Bailey TM, Brandt FH, et al: Acanthamoeba keratitis in soft contact lens wearers: A case-control study. J Am Med Assoc 258:57–60, 1987 181. Moore MB, McCulley JP, Newton C, et al: Acanthamoeba keratitis: A growing problem in soft and hard contact lens wearers. Ophthalmology 94:1654–1661, 1987 182. Koenig SB, Solomon JM, Hyndiuk RA, et al: Acanthamoeba keratitis associated with gas-permeable contact lens wear (Letter). Am J Ophthalmol 103:832, 1988 183. Heidemann DG, Verdier DD, Dunn SP, Stamler JF: Acanthamoeba keratitis associated with disposable contact lenses. Am J Ophthalmol 110:630–634, 1990 184. Doren GS, Cohen EJ, Higgins SE, et al: Management of contact lens associated acanthamoeba keratitis. CLAO J 17(2):120–125, 1991 185. Penley CA, Willis SW, Sickler SG: Comparative antimicrobial efficacy of soft and rigid gas permeable contact
lens solutions against acanthamoeba. CLAO J 15(4):257–260, 1989 186. Silvany RE, Dougherty JM, McCulley JP, et al: The effect of currently available contact lens disinfection systems on acanthamoeba castellanii and acanthamoeba polyphaga. Ophthalmology 97:286–290, 1990 187. Silvany RE, Dougherty JM, McCulley JP: Effect of contact lens preservatives on acanthamoeba. Ophthalmology 98:854–857, 1991 188. Ruiz-Montenegro J, Mafra CH, Wilson SE, et al: Corneal topographic alterations in normal contact lens wearers. Ophthalmol 100:128–134, 1993 189. Mobilia EF, Kenyon KR: Contact lens-induced corneal warpage. Int Ophthalmol Clin 26(1):43–53, 1986 190. Asbell PA, Wasserman D: Contact lens-induced corneal warpage. Int Ophthalmol Clin 31(2):121–126, 1991 191. Wilson SE, Lin DTC, Klyce SD, et al: Rigid contact lens decentration: A risk factor for corneal warpage. CLAO J 16(3):177–182, 1990 192. Phillips CI: Contact lenses and corneal deformation: Cause, correlate or coincidence? Acta Ophthalmol 68:661–668, 1990 193. Wilson SE, Lin DTC, Klyce SD, et al: Topographic changes in contact lens-induced corneal warpage. Ophthalmol 97:734–744, 1990 194. Krachmer JH, Feder RS, Belin MW: Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol 28:293–322, 1984 195. Nauheim JS, Perry HD: A clinicopathologic study of contact lens-related keratoconus. Am J Ophthalmol 100:543–546, 1985 196. Macsai MS, Varley GA, Krachmer JH: Development of keratoconus after contact lens wear: Patient characteristics. Arch Ophthalmol 108:534–538, 1990 197. MacRae SM, Matsuda M, Shellans S: Corneal endothelial changes associated with contact lens wear. CLAO J 15(1):82–87, 1989 198. Holden BA, Williams L, Zantos SG: The etiology of transient endothelial changes in the human cornea. Invest Ophthalmol Vis Sci 26:1354–1359, 1985 199. Barr JT, Schoessler JP: Corneal endothelial response to rigid contact lenses. Am J Optom Physiol Opt 57(5):267–274, 1980 200. Holden BA, Williams L, Sweeney DF, Swarbrick HA: The endothelial response to contact lens wear. CLAO J 12(3):150–152, 1986 201. Stocker EG, Schoessler JP: Corneal endothelial polymegathism induced by PMMA contact lens wear. Invest Ophthalmol Vis Sci 26(6):857–863, 1985 202. Hirst LW, Auer C, Cohn J, et al: Specular microscopy of hard contact lens wearers. Ophthalmology 91:1147–1153, 1984 203. Yamauchi K, Hirst LW, Enger C, et al: Specular microscopy of hard contact lens wearers II. Ophthalmology 96:1176–1179, 1989 204. Orsborn GN, Schoessler JP: Corneal endothelial polymegathism after the extended wear of rigid gas-permeable
contact lenses. Am J Optom Physiol Opt 65(2):84–90, 1988 205. Dada VK, Jain AK, Mehta MR: Specular microscopy of unilateral hard contact lens wearers. Indian J Ophthalmol 37(1):17–19, 1989 206. Matsuda M, Macrae SM, Inaba M, Manabe R: The effect of hard contact lens wear on the keratoconic corneal endothelium
after penetrating keratoplasty. Am J Ophthalmol 107:246–251, 1989 207. Sibug ME, Datiles MB, Kashima K, et al: Specular microscopy studies on the corneal endothelium after cessation
of contact lens wear. Cornea 10(5):395–401, 1991 208. MacRae SM, Matsuda M, Shellans S, Rich LF: The effects of hard and soft contact lenses on the corneal endothelium. Am J Ophthalmol 102:50–57, 1986 209. Lass JH, Dutt RM, Spurney RV, et al: Morphologic and fluorophotometric analysis of the corneal endothelium in
long-term hard and soft contact lens wearers. CLAO J 14(2):105–109, 1988 210. Carlson KH, Bourne WM, Brubaker RF: Effect of long-term contact lens wear on corneal endothelial cell
morphology and function. Invest Ophthalmol Vis Sci 29(2):185–193, 1988 211. Holden BA, Sweeney DF, Vannas A, et al: Effects of long-term extended contact lens wear on the human cornea. Invest Ophthalmol Vis Sci 26(11):1489–1501, 1985 212. Carlson KH, Bourne WM: Endothelial morphologic features and function after long-term extended
wear of contact lenses. Arch Ophthalmol 106:1677–1679, 1988 213. Matsuda M, Inaba M, Suda T, Macrae SM: Corneal endothelial changes associated with aphakic extended contact lens
wear. Arch Ophthalmol 106:70–72, 1988 214. Dutt RM, Stocker EG, Wolff CH, et al: A morphologic and fluorophotometric analysis of the corneal endothelium
in long-term extended wear soft contact lens wearers. CLAO J 15(2):121–123, 1989 |