- Diagnosing the presence of specific microbial pathogens that are the
likely cause of infectious disease in the eye.
- Detecting a predominance of certain cell types (e.g., eosinophils, macrophages, epithelial ingrowth, ghost erythrocytes, phacolytic
cells) that may provide a clue as to the etiology of an inflammatory
disease, which may be autoimmune or allergic in nature.
- Identifying:
- Specific antibodies in the aqueous humor or vitreous aspirate that are
suggestive of infection (e.g., Toxocara, Toxoplasma, herpesvirus, syphilis).
- Proteins (e.g., lens proteins, angiotensin-converting enzyme) that are suggestive
of granulomatous inflammation, such as sarcoidosis.
- Specific antibodies in the aqueous humor or vitreous aspirate that are
suggestive of infection (e.g., Toxocara, Toxoplasma, herpesvirus, syphilis).
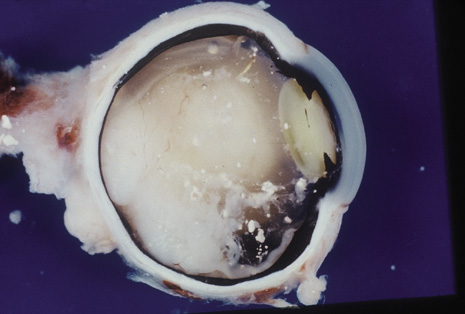
Immune complexes and antibodies associated with Behçet's disease may be found. Polymerase chain reaction (PCR) analysis has suggested the presence of DNA from the infection. Tumor cells may be identified when a malignant infiltration of the eye (e.g., large cell lymphoma, leukemia, retinoblastoma, malignant melanoma) masquerades as a uveitis, or by the presence of tumor cell enzymes and antigens (Table 1). 1,2
TABLE 1. Diagnostic Paracentesis
| Finding | Condition or Disease Indicated |
| Aqueous | |
| Bacteria | Endophthalmitis |
| Fungi | Candida, Aspergillus, etc. |
| Tumor cells | Retinoblastoma, malignant melanoma, reticulum cell sarcoma, leukemia, metastatic cancer |
| Eosinophils | Toxocara canis |
| Macrophages | Phacolytic glaucoma |
| Antibodies (ELISA) | Toxoplasma gondii, T. canis, reticular cell sarcoma, Behçet's disease, syphilis |
| Immune complexes | Behçet's disease |
| Other proteins | |
| Angiotensin-converting enzymes | Sarcoid |
| Lactate dehydrogenase isoenzymes | Retinoblastoma |
| Lens fragments | Phagocytolytic glaucoma |
| Ghost erythrocytes | Hemorrhagic glaucoma |
| Metastatic cancer cells | Metastatic cancer |
| JXG | JXG |
| Mesenchymal fibrous cells | PHPV |
| Amyloid | Amyloid |
| Epithelial cells | Epithelial ingrowth |
| Vitreous | |
| Bacteria | Endophthalmitis |
| Fungi | Candida, Aspergillus sp., etc. (e.g., cryptovirus) |
| Tumor cells | Retinoblastoma, malignant melanoma, reticular cell sarcoma, leukemia, metastatic cancer |
| Eosinophils | T. canis |
| Antibodies | T. gondii, reticular cell sarcoma, Behçet's disease, syphilis (and immune complexes) |
| Macrophages | Sympathetic ophthalmia, severe retinitis |
| Amyloid | Amyloid |
| Calcium soaps | Asteroid hyalosis |
| Polymerase chain reaction of viral DNA | CMV retinitis1,2 |
| Interleukin-10 (ELISA) | RCS3 |
| Monoclonal antibodies to Behçet's disease (long) | Behçet's disease (clinical activity, numbers of cells)4 |
| ?HLA DR and DRY | VKH (equally prevalent among Hispanics and Japanese)5 |
CMV = cytomegalovirus; ELISA = enzyme-linked immunosorbent assay; HLA = human leukocyte antigen.
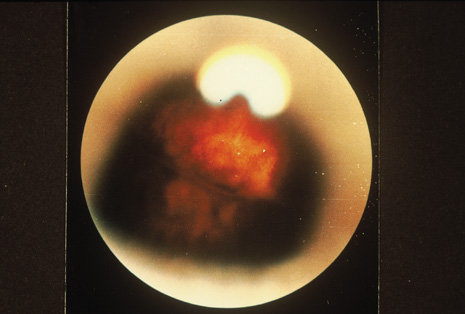
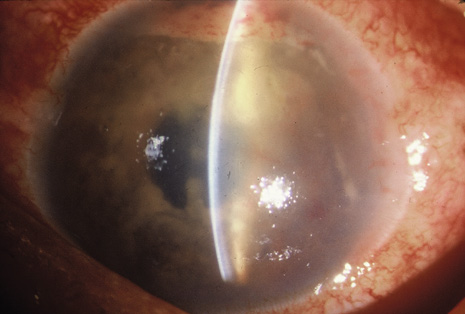
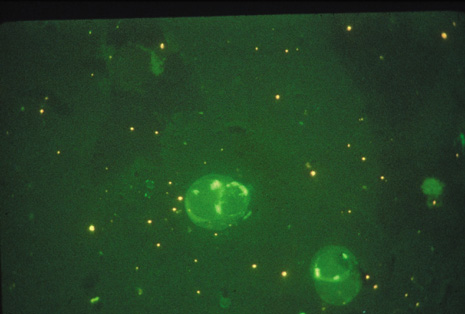
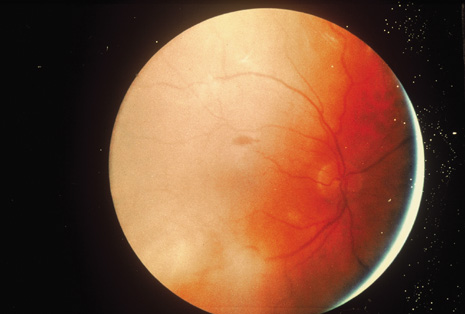
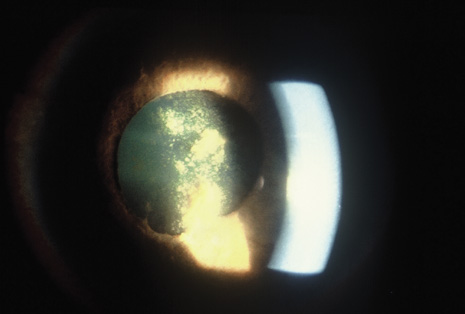
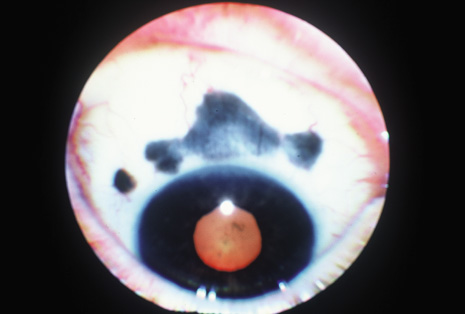
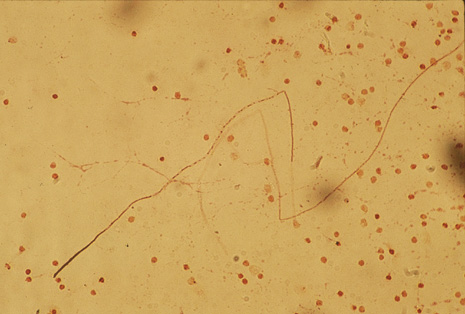
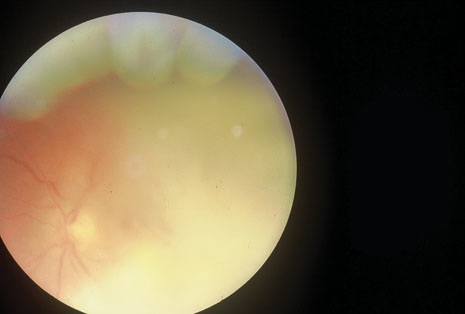
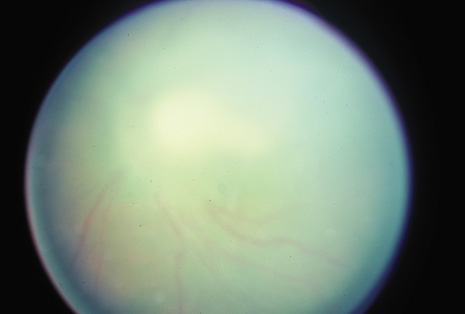
Although keratocentesis had been advocated historically as a treatment for active uveitis, it lost the attention of ophthalmologists until 1919, when Bruckner3 first examined the aqueous humor for diagnostic purposes. Laboratory techniques were revolutionized in the 20th century in areas such as: (a) evaluating very small aliquots of fluid (0.2 to 0.3 mL of aqueous or vitreous), and (b) identifying specific microbial organisms and the predominance of other cell types, antibodies, and proteins in these fluids (Figs. 1, 2, 3, 4, 5, and 6). These advancements have led to the development of diagnostic paracentesis for sight-threatening ocular inflammations that are difficult to diagnose. Witmer4 and O'Connor5 have provided strong evidence that samples of the aqueous humor reflect the antibody-producing capabilities of the iris and ciliary body, particularly when more specific antibody per unit of gamma globulin can be found on the aqueous humor than in the blood of the same patient.6–8 These determinations may be highly significant when one considers the fact that diseased tissue is being bathed in an antibody-containing fluid that is elaborated locally. For instance, in the case shown in Figure 1, the immunofluorescent antibody titer to toxoplasmosis is four times greater in the vitreous aspirate at the time of vitrectomy for repair of retinal detachment than in the plasma. These same considerations have long been recognized in syphilis of the central nervous system, wherein specific antibodies may be present in the cerebrospinal fluid but not in the blood. This is also the case with an unusual presentation of ocular coccidioidomycosis9 or toxocariasis.
|
|
|
|
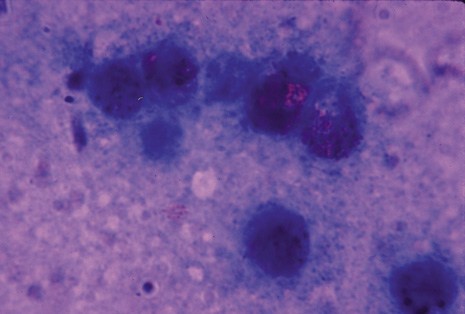
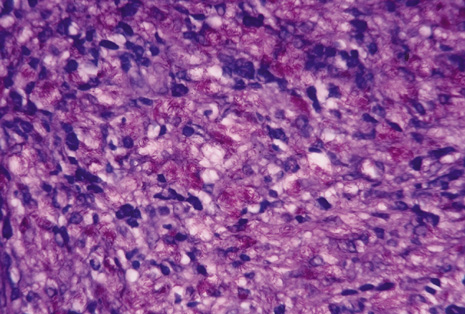
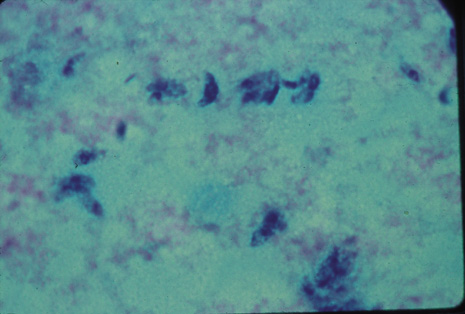
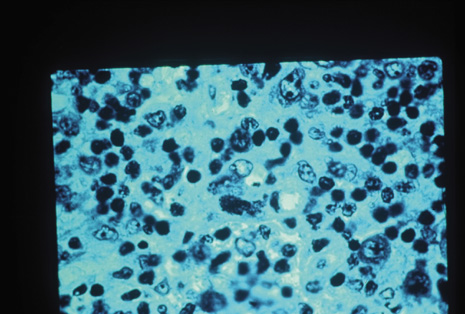
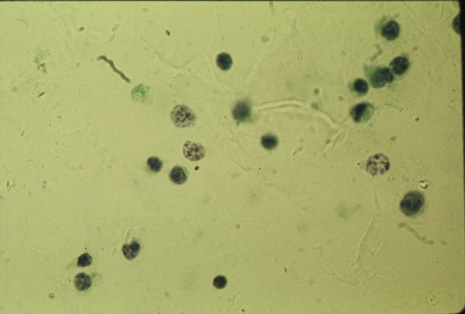
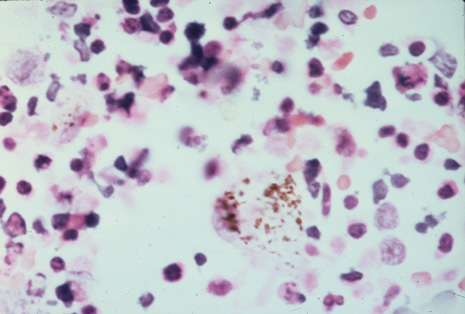
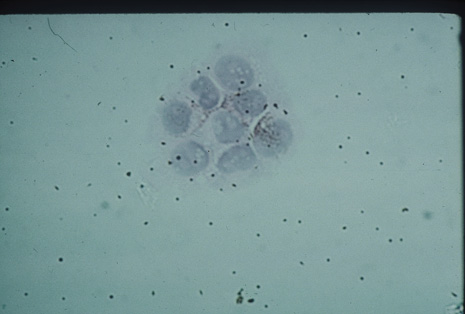
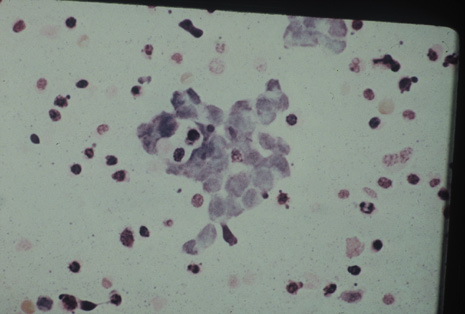
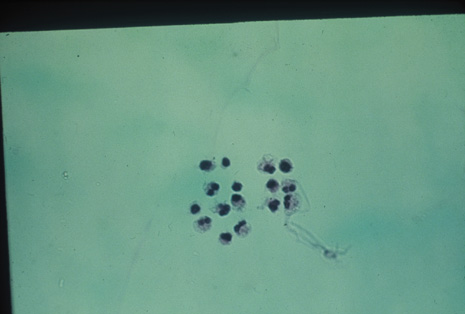
Many forms of uveitis are characterized by specific types of inflammatory cells. Usually, however, one encounters mixtures of cell types in any given specimen, with the relative percentages of lymphocytes and polymorphonuclear leukocytes varying. There may be unusual numbers of eosinophils, or macrophages laden with lens material may be present. Thus, an enumeration of the cells and a careful analysis of their structure can be useful as a diagnostic aid (Figs. 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, and 20). Figure 15 demonstrates eosinophils that were aspirated from the anterior chamber of a patient with Toxocara canis endophthalmitis. Figure 12 demonstrates malignant cell infiltrate from the vitreous, showing the stained presence of monoclonal light chains being elaborated in the cytoplasm. Interleukin-10, detectable in the vitreous of intraocular lymphoma patients, is also directly indicative of both the clinical activity and the number of malignant cells as observed by cytopathology.
|
|
|
|
|
|
|
|
|
|
|
|
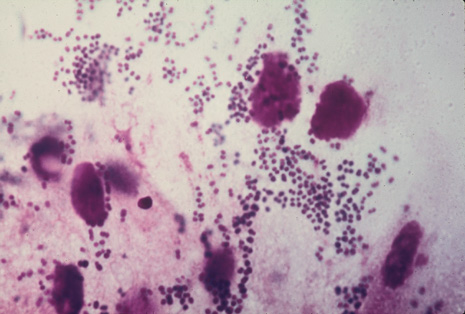
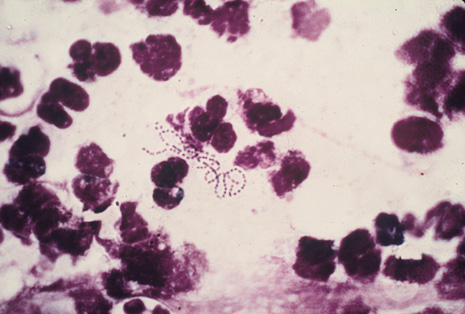
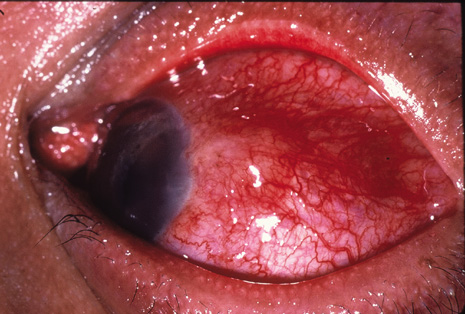
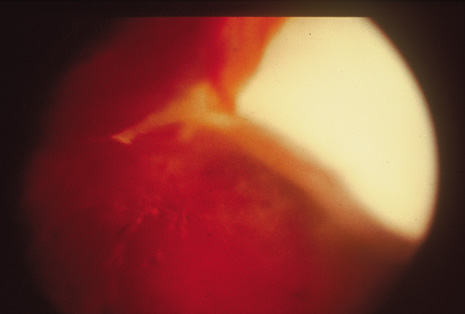
Precise identification and culture of bacterial and fungal pathogens from both the aqueous humor and the vitreous fluid can be obtained. Gram's stain and Giemsa's stain smears of centrifuged specimens from the aqueous humor and the vitreous humor frequently demonstrate the bacterial or fungal causative agent. Attempts to isolate bacteria and fungi and to identify them on Gram's stain or Giemsa's stain smears have been most rewarding in the following cases: (a) postoperative endophthalmitis, (b) infection after a penetrating injury of the eye, (c) drug abuse patients with endogenous endophthalmitis (Figs. 21, 22, 23, 24, and 25), (d) patients receiving hyperalimentation, and (4) patients who are immunocompromised as a result of exogenous immunosuppressive agents.
|
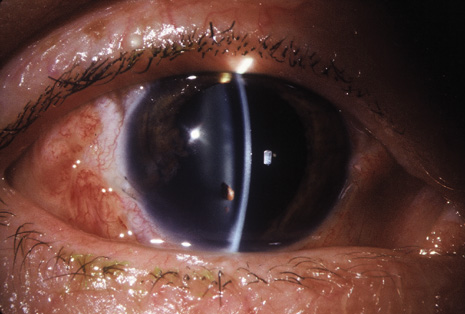
Studies have demonstrated the usefulness of ocular paracentesis for the identification of ocular infections in order to implement sight-saving treatment.10–16 Even acid-fast bacilli and viruses may be diagnosed in this fashion when emergency dictates (see Fig. 5).17 It is recommended that diagnostic paracentesis be performed in all cases of postoperative endophthalmitis, and it is safe to perform the postoperative procedure in the operating room with the safety of vitrectomy surgery. Further, any patient older than 65 who presents with a deteriorating uveitis (usually with vitreitis as the predominant infiltrate) of undetermined etiology should undergo paracentesis of the vitreous to rule out reticulum cell sarcoma (large cell lymphoma).18 Similarly, any patient suspected of being an intravenous drug abuser who presents with an endogenous endophthalmitis or uveitis should undergo diagnostic paracentesis to avoid allowing an intraocular infection to be borne by the bloodstream.19,20