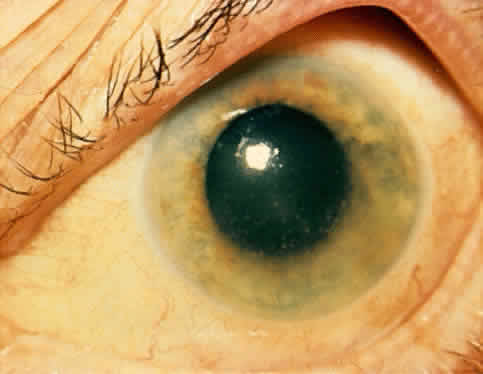
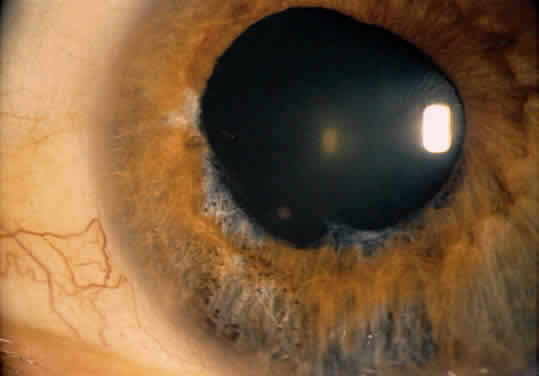
1. Hogan MJ, Kimura SJ, Thygeson P: Signs and symptoms of uveitis: I. Anterior uveitis. Am J Ophthalmol 47:155, 1959 2. Cassidy JT, Petty RE: Juvenile rheumatoid arthritis. In Cassidy JT, Petty
RE (eds): Textbook of Pediatric Rheumatology, p 133. Philadelphia, WB
Saunders, 1995 3. Cassidy JT, Brody GL, Martel W: Monarticular juvenile rheumatoid arthritis. J Pediatr 70:867, 1967 4. Bywaters EGL, Ansell BM: Monarticular arthritis in children. Am Rheum Dis 24:116, 1965 5. Lindsley CB: Pauciarticular JRA. In Miller JJ III (ed): Juvenile Rheumatoid
Arthritis, p 135. Littleton, MA, PSG Publishing Co, 1979 6. Lindsley CB: Juvenile rheumatoid arthritis and spondyloarthropathies. Curr Opin Rheumatol 7:425, 1995 7. Haas JR, Truckenbrodt H, Paul C et al: Subtypes of HLA-DRB1*03, *08, *11, *12, *13 and *14 in early onset pauciarticular
juvenile chronic arthritis (EOPA) with and without iridocyclitis. Clin Exp Rheumatol 12(suppl 10):57, 1994 8. Bywaters EGL: Still's disease in the adult. Ann Rheum Dis 30:121, 1971 9. Key SN, Kimura SJ: Iridocyclitis associated with juvenile rheumatoid arthritis. Am J Ophthalmol 80:425, 1975 10. Kanski JJ: Anterior uveitis in juvenile rheumatoid arthritis. Arch Ophthalmol 95:1974, 1977 11. Kanski JJ, Shun-Shen A: Systemic uveitis in childhood: an analysis of 340 cases. Ophthalmology 91:1247, 1984 12. Schaller JG, Johnson GD, Holborow EJ et al: The association of antinuclear antibodies with chronic iridocyclitis of
juvenile rheumatoid arthritis (Still's disease). Arthritis Rheum 17:409, 1974 13. Chylack LT, Bienfang DC, Bellows AR et al: Ocular manifestations of juvenile rheumatoid arthritis. Am J Ophthalmol 79:1025, 1975 14. Smiley WK: The eye in juvenile rheumatoid arthritis. Trans Ophthalmol Soc UK 94:817, 1974 15. Schaller J, Kupfer C, Wedgewood RJ: Iridocyclitis in juvenile rheumatoid arthritis. Pediatrics 44:92, 1969 16. Rosenberg DM, Oen KG: The relationship between ocular and articular disease activity in children
with juvenile rheumatoid arthritis and associated uveitis. Arthritis Rheum 29:797, 1986 17. Petty RE: Current knowledge of the etiology and pathogenesis of chronic uveitis accompanying
juvenile rheumatoid arthritis. Rheum Dis Clin North Am 13:19, 1987 18. Praeger DL, Schneider HA, Sakowski AD et al: Kelman procedure in the treatment of complicated cataract of the uveitis
of Still's disease. Trans Ophthalmol Soc UK 96: 168, 1976 19. Cabral DA, Petty RE, Malleson RN: Visual prognosis in children with chronic uveitis and arthritis. Arthritis Rheum 35:5229, 1992 20. Olson NY, Lindsley CB, Godfrey WA: Effects of nonsteroidal anti-inflammatory drugs on chronic childhood iridocyclitis. J Allergy Clin Immunol 79:220, 1987 21. Lang BA, Finlayson LA: Naproxen-induced pseudoporphyria in patients with juvenile rheumatoid arthritis. J Pediatr 124:639, 1994 22. Wallace CA, Farrow D, Sherry DD: Increased risk of facial scars in children taking nonsteroidal anti-inflammatory
drugs. J Pediatr 125:819, 1994 23. Paulus HE: FDA arthritis advisory committee meeting: juvenile rheumatoid arthritis
drug evaluation guidelines for over the counter naproxen. Arthritis Rheum 37:137, 1994 24. Kordonouri O, Dracou C, Papadelles F et al: Glomerular microproteinuria in children treated with nonsteroidal anti-inflammatory
drugs for juvenile chronic arthritis. Clin Exp Rheumatol 12:561, 1994 25. Kimura SJ, Hogan MJ, O'Connor GR et al: Uveitis and joint disease: clinical findings in 191 cases. Arch Ophthalmol 77:309, 1967 26. Perkins ES: Patterns of uveitis in children. Br J Ophthalmol 50:169, 1966 27. Kimura SJ, Hogan MJ, Thygeson P: Fuchs' syndrome of heterochromic cyclitis. Arch Ophthalmol 54:179, 1955 28. Loewenfeld IE, Thompson HS: Fuchs' heterochromic cyclitis: a critical review of the literature: I. Clinical
characteristics of the syndrome. Surv Ophthalmol 17:394, 1973 29. Loewenfeld IE, Thompson HS: Fuchs' heterochromic cyclitis: a critical review of the literature: II. Etiology
and mechanisms. Surv Ophthalmol 18:2, 1973 30. O'Connor GR: Heterochromic iridocyclitis (Doyne lecture). Trans Ophthalmol Soc UK 104:219, 1985 31. deAbreu MT, Belfort R, Hirata PS: Fuchs' heterochromic cyclitis and ocular toxoplasmosis. Am J Ophthalmol 93: 739, 1982 32. Arffa RC, Schlaegel TF: Chorioretinal scars in Fuchs' heterochromic iridocyclitis. Arch Ophthalmol 102:1153, 1984 33. Schwab IR: The epidemiologic association of Fuchs' heterochromic iridocyclitis and
ocular toxoplasmosis. Am J Ophthalmol 111:356, 1991 34. Le Heij E, Rothova A, Baarsma GS et al: Fuchs' heterochromic iridocyclitis is not associated with ocular toxoplasmosis. Arch Ophthalmol 110:806, 1992 35. Goble RR, Murray PI: Fuchs' heterochromic uveitis and sarcoidosis. Br J Ophthalmol 79:1021, 1995 36. Kimura SJ: Fuchs' syndrome of heterochromic cyclitis in brown-eyed patients. Trans Am Ophthalmol Soc 76:76, 1978 37. Tabbut BR, Tessler HH, Williams D: Fuchs' heterochromic iridocyclitis in blacks. Arch Ophthalmol 106:1688, 1988 38. Makley TA: Heterochromic cyclitis in identical twins. Am J Ophthalmol 41:768, 1956 39. Saari M, Vuorre I, Nieminen H: Fuchs' heterochromic cyclitis: a simultaneous bilateral fluorescein angiography
study of the iris. Br J Ophthalmol 62:715, 1978 40. Berger BB, Tessler HH, Kottow MH: Anterior segment ischemia in Fuchs' heterochromic cyclitis. Arch Ophthalmol 98:499, 1980 41. Perry HD, Yanoff M, Scheie HG: Rubeosis in Fuchs' heterochromic iridocyclitis. Arch Ophthalmol 93:337, 1975 42. Amsler M, Verrey F: Heterochromie de Fuchs et fragilité vasculaire. Ophthalmologica 111:177, 1946 43. Jones NP: Glaucoma in Fuchs' heterochromic uveitis: etiology, management and outcome. Eye 5:662, 1991 44. Leisegang TJ: Clinical features and prognosis in Fuchs' uveitis syndrome. Arch Ophthalmol 100:1622, 1982 45. Epstein DL: Glaucoma due to intraocular inflammation. In Epstein DL (ed): Chandler
and Grant Glaucoma, p 365. Philadelphia, Lea & Febiger, 1986 46. Smith RE, O'Connor GR: Cataract extraction in Fuchs' syndrome. Arch Ophthalmol 91:39, 1974 47. Franceschetti A: Heterochromic cyclitis (Fuchs' syndrome). Am J Ophthalmol 39:50, 1955 48. Jones NP: Extracapsular cataract surgery with and without intraocular implantation
in Fuchs' heterochromic uveitis. Eye 4:145, 1990 49. Baarsma GS, deVries J, Hammudogler CD: Extracapsular cataract extraction with posterior chamber lens implantation
in Fuchs' heterochromic cyclitis. Br J Ophthalmol 75:306, 1991 50. Jakeman CM, Jordan K, Keast-Gutler J, Perry C: Cataract surgery with intraocular lens implantation in Fuchs' heterochromic
cyclitis. Eye 4:543, 1990 51. Gee SS, Tabbara KF: Extracapsular cataract extraction in Fuchs' heterochromic iridocyclitis. Am J Ophthalmol 108:310, 1989 52. Murray PI, Mooy CM, Visser-deJong E et al: Immunohistochemical analysis of iris biopsy specimens from patients with
Fuchs' heterochromic cyclitis. Am J Ophthalmol 109:394, 1990 53. Jones NP: Fuchs' heterochromic uveitis: a reappraisal of the clinical spectrum. Eye 5:649, 1991 54. Schwab IR: Fuchs' heterochromic iridocyclitis. Int Ophthalmol Clin 30:252, 1990 55. McCartney ACE, Bull TB, Spalton DJ: Fuchs' heterochromic cyclitis: an electron microscopic study. Trans Ophthalmol Soc UK 105:324, 1986 56. Chaves AD: Prevalence and demographic characteristics of sarcoidosis in
New York City. In Turiaf J, Chabot J (eds): La Sarcoidose. Paris, Masson, 1967 57. Hagerstrad I, Linell F: Prevalence of sarcoidosis in the autopsy material from a Swedish town. Acta Med Scand 176(suppl 425):171, 1964 58. James DG, Jones W: Ocular and neurosarcoidosis. In: Sarcoidosis and Other
Granulomatous Disorders. Philadelphia, WB Saunders, 1985 59. Parada NA, Center DM, Berman JS: Sarcoidosis. In Rich RR (ed): Clinical
Immunology Principles and Practice, p 1250. St. Louis, Mosby, 1996 60. Sharma O: Sarcoidosis. Dis Mon 36:460, 1990 61. Thomas P, Hunninglake G: Current concepts of the pathogenesis of sarcoidosis. Am Rev Respir Dis 135:747, 1987 62. Weinberg RS, Tessler HH: Serum lysozyme in sarcoid uveitis. Am J Ophthalmol 82:105, 1976 63. Weinreb RN, Barth R, Kimura SJ: Limited gallium scans and angiotensin converting enzyme in granulomatous
uveitis. Ophthalmology 87:202, 1980 64. Weinreb RN, Tessler HH: Laboratory diagnosis of ophthalmic sarcoidoses. Surv Ophthalmol 28:653, 1984 65. Jabs DA, Johns CJ: Ocular involvement in chronic sarcoidosis. Am J Ophthalmol 102:297, 1986 66. Brockhurst RJ, Schepens CL, Okamura ID: Uveitis: II. Peripheral uveitis: clinical description, complications and
differential diagnosis. Am J Ophthalmol 49:1257, 1960 67. Welch RB, Maumenee AE, Wahlen HE: Peripheral posterior segment inflammation, vitreous opacities and edema
of the posterior pole: pars planitis. Arch Ophthalmol 64:540, 1960 68. Kimura SJ, Hogan MJ: Chronic cyclitis. Arch Ophthalmol 71:193, 1964 69. Smith RE, Godfrey WA, Kimura SJ: Chronic cyclitis: I. Course and visual prognosis. Trans Am Acad Ophthalmol Otolaryngol 77:760, 1973 70. Smith RE, Godfrey WA, Kimura SJ: Complications of chronic cyclitis. Am J Ophthalmol 82:277, 1976 71. Pruett RC, Brockhurst RJ, Letts NF: Fluorescein angiography of peripheral uveitis. Am J Ophthalmol 77:448, 1974 72. Pederson JE, Kenyon KR, Green WR et al: Pathology of pars planitis. Am J Ophthalmol 86:762, 1978 73. Saguerg TM, Cesarz TJ, Flicklinger RR: Treatment of pars planitis: I. Cryotherapy. Surv Ophthalmol 22:120, 1972 74. Giles CL: Pediatric intermediate uveitis. J Pediatr Ophthalmol Strabismus 26:136, 1989 75. Knox DL: Ischemic ocular inflammation. Am J Ophthalmol 60:995, 1965 76. Jacobs NA, Ridgway AEA: Syndrome of ischemic ocular inflammation: six cases and a review. Br J Ophthalmol 69:681, 1985 77. Hunder GG, Hazelman BL: Giant cell arteritis and polymyalgia rheumatica. In
Kelley WN, Harris ED, Ruddy S et al (eds): A Textbook of Rheumatology, p 1166. Philadelphia, WB Saunders, 1985 78. Healey LA: Polymyalgia rheumatica and giant cell arteritis. In Wyngaarden
JB, Smith SH (eds): Cecil Textbook of Medicine, p 1946. Philadelphia, WB
Saunders, 1985 79. Hedges TR: The aortic arch syndromes. Arch Ophthalmol 71:28, 1964 80. Conn DL, Hunder GG: Necrotizing vasculitis. In Kelley WN, Harris ED, Ruddy
S et al (eds): A Textbook of Rheumatology, p 1137. Philadelphia, WB
Saunders, 1985 81. O'Connor GR: Recurrent herpes simplex uveitis in humans. Surv Ophthalmol 21:165, 1976 82. Wilhelmuts KR, Falcon MG, Jones BR: Herpetic iridocyclitis. Int Ophthalmol 4:143, 1982 83. Tokumaru T, Wilentz J: Iridoplegia and aqueous flare due to acute herpetic keratouveitis. Can J Ophthalmol 10: 193, 1975 84. Naumann G, Gass JDM, Font RL: Histopathology of herpes zoster ophthalmicus. Am J Ophthalmol 65:533, 1968 85. Marsh RJ, Easty DL, Jones BR: Iritis and iris atrophy in herpes zoster ophthalmicus. Am J Ophthalmol 78:255, 1974 86. Womack LW, Leisegang TJ: Complications of herpes zoster ophthalmicus. Arch Ophthalmol 101:42, 1983 87. Cobo M, Foulks GN, Leisegang TJ et al: Oral acyclovir in the therapy of acute herpes zoster ophthalmicus. Ophthalmology 92:1574, 1985 88. Aronson SB, Elliott JH: Ocular Inflammations, p 223. St. Louis, CV Mosby, 1972 89. Denis-Lassalle J: Viral uveitis. In Campinchi R, Faure JP, Bloch-Michel
E et al (eds): Uveitis: Immunologic and Allergic Phenomena, p 498 [Golden
B, Givoiset MM transl]. Springfield, IL, Charles C Thomas, 1973 90. Kenyon KR: Ocular involvement associated with chronic Epstein-Barr viral disease. Arch Ophthalmol 105:788, 1987 91. Martinet AC: Role of viruses in uveitis. Trans Ophthalmol Soc UK 101:308, 1981 92. Colvard DM, Robertson DM, O'Duffy JD: The ocular manifestations of Behçet's disease. Arch Ophthalmol 95:1813, 1977 93. Michelson JB, Chisari FV: Behçet's disease. Surv Ophthalmol 26:190, 1982 94. Ohno S, Char DH, Kimura SJ et al: Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 83:735, 1977 95. Snyder DA, Tessler HH: Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol 90:69, 1980 96. Kanter PJ, Goldberg MF: Bilateral uveitis with exudative retinal detachment: angiographic appearance. Arch Ophthalmol 91:13, 1974 97. Green WR: Uveal tract. In Spencer WH (ed): Ophthalmic Pathology: An Atlas
and Textbook, Vol 3, p 1352. Philadelphia, WB Saunders, 1986 98. Lubin JR, Albert DM, Weinstein M: Sixty-five years of sympathetic ophthalmia: a clinicopathologic review
of 105 cases (1913-1978). Ophthalmology 87:109, 1980 99. Marak GE Jr, Font RL, Alepa FP: Experimental lens-induced granulomatous endophthalmitis. Mod Probl Ophthalmol 16:75, 1976 100. Rahi AHS, Misra RN, Morgan G: Immunopathology of the lens: III. Humoral and cellular immune responses
to autologous lens antigens and their role in ocular inflammation. Br J Ophthalmol 61:371, 1977 101. Marak GE, Rao NA: Lens protein binding lymphocytes. Ophthalmic Res 15:6, 1983 102. Marak GE Jr, Rao NA, Scott JM et al: Antioxidant modulation of phacoanaphylactic endophthalmitis. Ophthalmic Res 17:197, 1985 103. Bloch-Michel E: Autoimmunity to the lens. In Campinchi R, Faure JP, Bloch-Michel
E et al (eds): Uveitis: Immunologic and Allergic Phenomena, p 102 [Golden
B, Givoiset MM transl]. Springfield, IL, Charles
C Thomas, 1973 104. Apple DJ, Mamalis M, Steinmetz RL et al: Phacoanaphylactic endophthalmitis associated with extracapsular cataract
extraction and posterior chamber intraocular lens. Arch Ophthalmol 102:1528, 1984 105. Blodi FC: Sympathetic uveitis as an allergic phenomenon with a study of its association
with phacoanaphylactic uveitis and a report on the pathological
findings in sympathizing eyes. Trans Am Acad Ophthalmol Otolaryngol 63:642, 1959 106. Spencer WH: Lens-induced inflammation. In Spencer WH (ed): Ophthalmic Pathology, Vol 1, p 473. Philadelphia, WB Saunders, 1985 107. Roussel TJ, Culbertson WW, Jaffe NS: Chronic post operative endophthalmitis associated with Propionibacterium acnes. Arch Ophthalmol 105:1199, 1987 108. Verstad SL, Price PK, Halsland KR, Kaiser RB: Phacolytic glaucoma after extracapsular cataract extraction. Glaucoma 5:206, 1983 109. Metzler DW: Sterile hypopyon following intraocular lens surgery. Arch Ophthalmol 98:100, 1980 110. Ellingson FT: The uveitis-glaucoma-hyphema syndrome associated with the Mark VIII anterior
chamber lens implant. J Am Implant Soc 4:50, 1978 111. Meisler DM, Mandelbaum S: Propionibacterium-associated endophthalmitis after extracapsular cataract extraction: review
of reported cases. Ophthalmology 96:54, 1989 112. Knox DL, Bayless TM, Yardley JH et al: Whipple's disease presenting with ocular inflammation and minimal
intestinal symptoms. Johns Hopkins Med J 123:175, 1968 113. Font RL, Rao NW, Issarescu S et al: Ocular involvement in Whipple's disease: light and electron microscopic
observations. Arch Ophthalmol 96:1431, 1978 114. Relman DA, Schmidt TM, MacDermott RP, Falkow S: Identification of the uncultured bacillus of Whipple's disease. N Engl J Med 327:293, 1992 115. Klinath RD et al: Antibiotic treatment and relapse in Whipple's disease: long term follow
up of 88 patients. Gastroenterology 88:1867, 1987 116. Good AE, Utsinger PD: Enteropathic arthritis. In Kelley WN, Harris ED, Ruddy
W et al (eds): Textbook of Rheumatology, p 1031. Philadelphia, WB
Saunders, 1985 117. Schlaegel TF: Essentials of Uveitis, p 90. Boston, Little, Brown & Co, 1969 118. Spink WW: Brucella. In Samiy AH, Smith LH Jr, Wyngaarden JB (eds): International
Textbook of Medicine, Vol 2, Medical Microbiology and Infectious
Disease, p 374. Philadelphia, WB Saunders, 1981 119. Bloch-Michel E: Uveitis caused by syphilis. In Campinchi R, Faure JP, Bloch-Michel
E et al (eds): Uveitis: Immunologic and Allergic Phenomena, p 390 [Golden
B, Givoiset MM transl]. Springfield, IL, Charles
C Thomas, 1973 120. Ross WH, Sutton HFS: Acquired syphilitic uveitis. Arch Ophthalmol 98:496, 1980 121. von Noorden GK, Buck AA: Ocular onchocerciasis. Arch Ophthalmol 80:26, 1968 122. Ben-Sira I, Yassur Y: Ocular onchocerciasis in Malawi. Br J Ophthalmol 56:617, 1972 123. Taylor HR, Dax EM: Onchocerciasis. In Tabbara KF, Hyndiuk RA (eds): Infections
of the Eye, p 653. Boston, Little, Brown & Co, 1986 124. Campinchi R: Uveitis of helminthiasis. In Campinchi R, Faure JP, Bloch-Michel
E et al (eds): Uveitis: Immunologic and Allergic Phenomena, p 452 [Golden
B, Givoiset MM transl]. Springfield, IL, Charles
C Thomas, 1973 125. Mandelbaum S, Forster RK, Gelender H et al: Late onset endophthalmitis associated with filtering blebs. Ophthalmology 92:964, 1985 126. Confino J, Brown SI: Bacterial endophthalmitis associated with exposed monofilament sutures
following corneal transplantation. Am J Ophthalmol 99:111, 1985 127. Gamel JW, Allansmith MR: Metastatic staphylococcal endophthalmitis presenting as chronic iridocyclitis. Am J Ophthalmol 77:454, 1974 128. Theodore FH: Etiology and diagnosis of fungal postoperative endophthalmitis. Ophthalmology 85:327, 1978 129. Pettit TH, Olson RJ, Foss RY et al: Fungal endophthalmitis following intraocular lens implantation. Arch Ophthalmol 98:1025, 1980 130. Forster RK: Etiology and diagnosis of bacterial postoperative endophthalmitis. Ophthalmology 85:320, 1978 131. Rowsey JJ, Newsom DL, Sexton DJ et al: Endophthalmitis: current approaches. Ophthalmology 89:1055, 1982 132. Shields JA, Shields CL: Endogenous endophthalmitis simulating retinoblastoma. Retina 15:213, 1995 133. Foster CS: Editorial: Vitrectomy in the management of uveitis. Ophthalmology 95:1111, 1988 134. Foster CS, Fong LP, Singh G: Cataract surgery and intraocular lens implantation in patients with uveitis. Ophthalmology 96:281, 1989 135. Kaplan HJ: Discussion of “Cataract surgery and intraocular lens implantation
in patients with uveitis.”Ophthalmology 96:281, 1984 136. Dangel ME, Stark WJ, Michels RG: Surgical management of cataract associated with chronic uveitis. Ophthalmic Surg 14:145, 1983 137. Molthenius PAT, Duetman AF: Surgical treatment of the complications of chronic uveitis. Ophthalmologica 186:11, 1983 138. Michelson JB, Friedlander MH, Nozik RA: Lens implant surgery in pars planitis. Ophthalmology 97:1023, 1990 139. Hooper PL, Rao NA, Smith RE: Cataract surgery in uveitis patients. Surv Ophthalmol 35:120, 1990 140. Kanski JJ: Lensectomy for complicated cataract in juvenile chronic iridocyclitis. Br J Ophthalmol 76:72, 1992 141. Tessler HH, Farber MD: Intraocular lens implantation versus no intraocular lens implantation in
patients with chronic iridocyclitis and pars planitis: a randomized
prospective study. Ophthalmology 100:1206, 1993 142. Foster CS, Barrett F: Cataract development and cataract surgery in patients with juvenile rheumatoid
arthritis-associated iridocyclitis. Ophthalmology 100:809, 1993 143. Probst LE, Holland EJ: Intraocular lens implantation in patients with juvenile rheumatoid arthritis-associated
uveitis. Am J Ophthalmol 122:161, 1996 144. Holland GN: Editorial: Intraocular lens transplantation in patients with juvenile rheumatoid
arthritis-associated uveitis: an unresolved management issue. Am J Ophthalmol 122:255, 1996 |