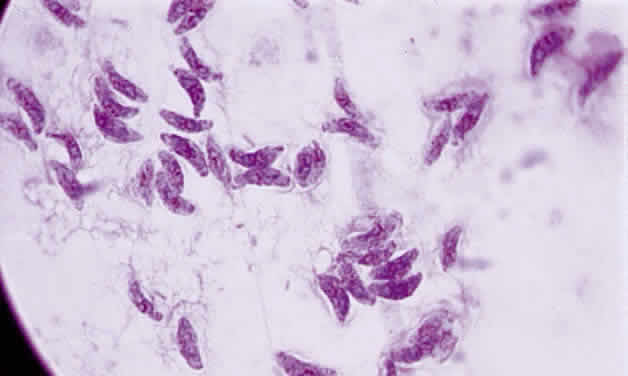
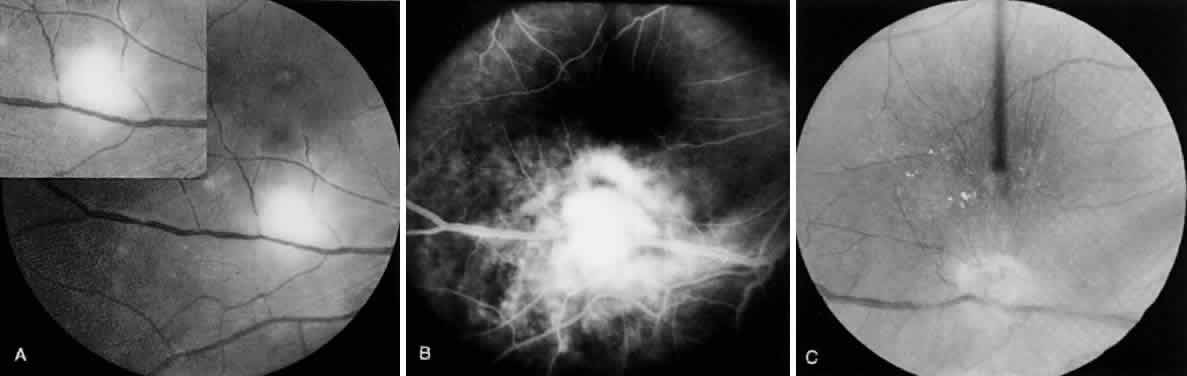
Tachyzoite is the proliferative form of the organism, which used to be known as trophozoite. It has a crescent shape and measures approximately 7 μm in length and 3 μm in width (Fig. 1). The organism is motile with a unique cytoskeletal structure allowing it to twist, wiggle, rotate, and glide. The rostrum of the tachyzoite is known as the conoid, which can extend, retract, tilt, and rotate. These movements allow the tachyzoite to find its target host cell and to penetrate the cell, establishing an intracellular existence. Tachyzoites are easily propagated in peritoneal cavities of mice and in mammalian tissue culture cell lines. The organism has the ability to replicate in all mammalian nucleated cells. In the host cell, the tachyzoite multiplies by endodyogeny; this reproductive process is susceptible to heat, freezing and thawing, desiccation, and gastric enzymes.
|
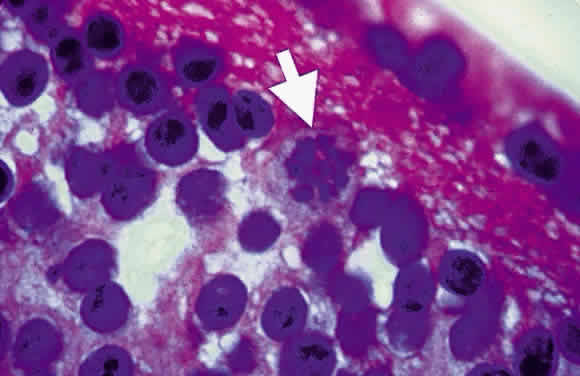
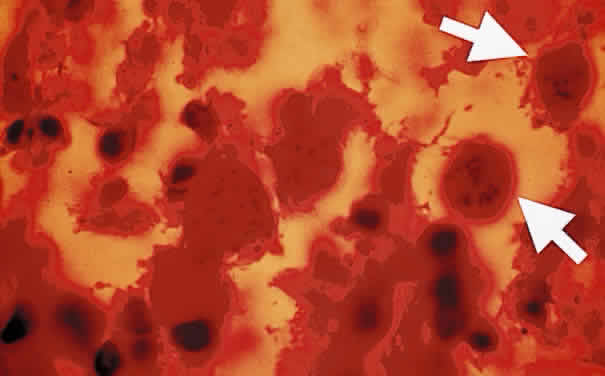
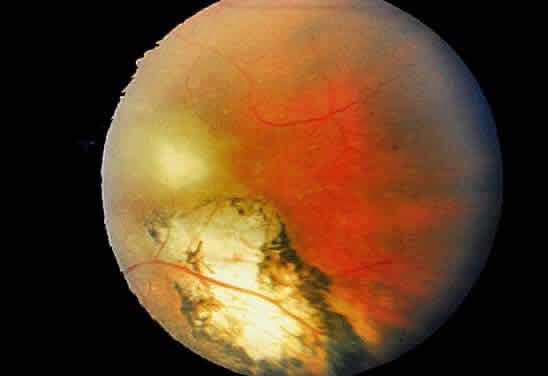
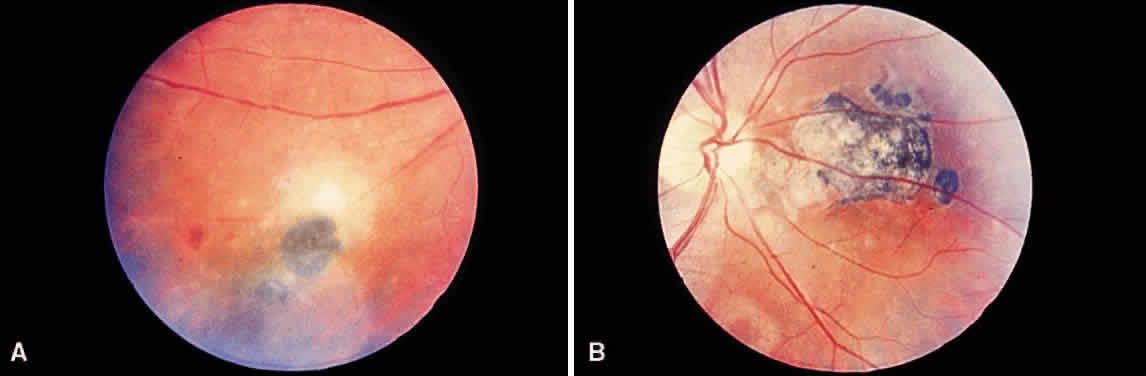
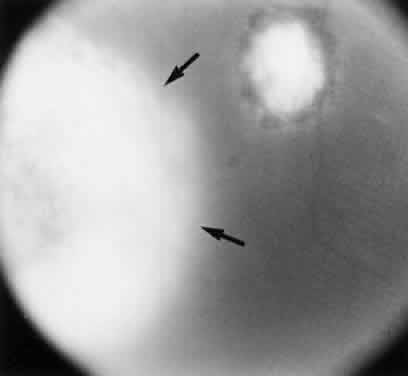
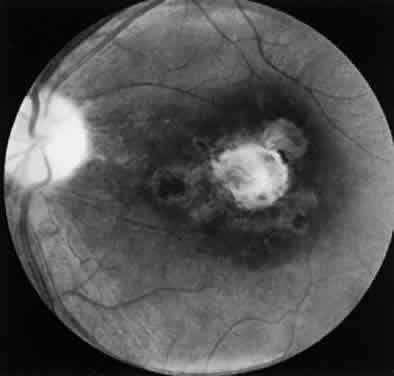
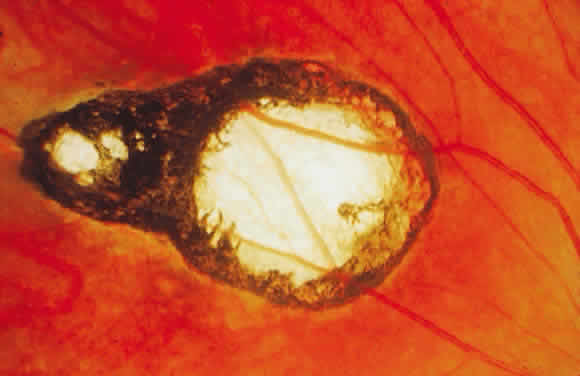
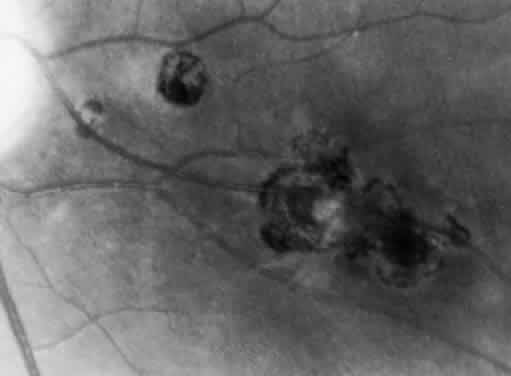
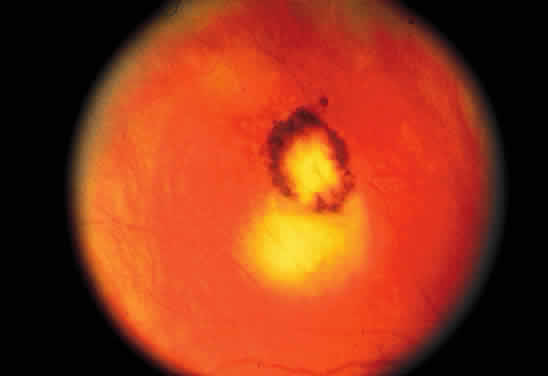
Bradyzoites are slowly metabolizing organisms found in cysts formed within the tissue of the infected host. The size of the Toxoplasma cyst varies, depending on the number of organisms that have multiplied within it. The cyst may reach more than 100 μm in diameter and may contain from 50 to 3000 organisms. The cyst wall is strongly argyrophilic and periodic acid-Schiff positive. It contains constituents that are derived from both the parasite and the host tissue. Constituent from the host tissue compose the outer part of the cyst, whereas those derived from the parasite are in the inner part of the cyst wall. Toxoplasmosis may be found in the inner layers of the retina after episodes of acute retinochoroiditis. The cyst may stay in the retinal tissue for years without showing any signs of invasiveness. Considering that the tissue cyst incorporates elements derived from the host into its outer wall, it is easily tolerated by the host, and no inflammatory reaction is seen around it (Fig. 2). It may remain for years in certain tissues, such as the eye or muscles, without provoking any inflammatory reactions. The bradyzoite inside the cyst derives its nutrition from the slow diffusion of substances through the cyst wall. The number of organisms increases within the cyst in the retina, and once the cyst wall breaks down by mechanical stretching, the bradyzoites escape, convert into tachyzoites, and invade contiguous cells. This process may lead to recurrence of retinitis. Certain immunologic mechanisms of the host may influence the organisms significantly. Immunosuppression coinciding with the rupture of the cyst and release of bradyzoites allows the organisms to become tachyzoites and proliferate in host tissue without restriction. The cyst of the Toxoplasma organism appears to be a defensive stage in its life cycle. The resistance of toxoplasmosis within chronically infected tissues of animals may lead to transmission of the disease by the ingestion of undercooked meat, including mutton, beef, pork, and chicken. Tissue cysts can develop within any organ and are commonly found in infected tissues of brain, eye, heart, skeletal muscles, and lymph nodes. Rupture of tissue cysts causes reactivation of the systemic toxoplasmosis in immune deficiency states, leading to dissemination of Toxoplasma organisms to other organs.
Sporozoites are found in the oocyst. Each sporozoite measures 10 to 12 μm in diameter. The sporozoite is produced in the intestines during the enteroepithelial part of the Toxoplasma life cycle. Toxoplasma organisms divide by endodyogeny (asexual reproduction), schizogony (splitting of nuclear material), and sexual reproduction involving the gametocytes. The gametocytes are found throughout the small intestines, especially in the ileum of the definitive host. The male gametocytes produce microgametes, which have the ability to fertilize macrogametes. After fertilization of the macrogamete, the zygote becomes surrounded by an oocyst wall and is shed in the feces of infected cats. Sporogony, which spans approximately 3 to 21 days, occurs within the oocyst outside of the host.1 The sporulated oocysts become infectious. Millions of oocysts may be excreted in the feces of an infected cat. Under conditions of moist and warm soil, the oocyst may remain infectious for more than a year.2 The oocyst appears to be an important method for the transmission of toxoplasmosis. Once ingested, the oocyst wall is digested by the gastric enzymes, trypsin and pepsin, thereby liberating Toxoplasma sporozoites. Shedding of the oocyst begins 1 to 24 days after the ingestion of the parasite by the cat. Sporulation requires 2 to 3 days at 24°C and 14 to 21 days at 11°C and does not occur above 37°C. Dry heat and exposure to temperatures above 66°C render the oocyst noninfectious. Puppies are susceptible to infection with Toxoplasma organisms and tend to shed more oocysts than adult cats.3 The reinfection of cats by Toxoplasma rarely leads to excretion of oocyst.
PENETRATION OF HOST CELL
Since the Toxoplasma organism is an obligate intracellular parasite, it cannot multiply in the extracellular space, and the initial step of entry into the host cell is critical for survival of the organism and the pathogenesis of the disease. The tachyzoite has a well-defined nucleus that is oval or round with a central karyosome. It is able to attain intracellular habitat by active invasion of host cell membrane. This is accomplished by a series of sequential steps involving the release of penetration enhancing factors. The conoid at the rostral end has the ability to thrust outward at time of invasion into the cell. At the time of contact between toxoplasmic plasmalemma and the host cell membrane, the differentiated organelles in the conoid, known as rhoptries, appear to play a role in the parasite penetration of the host cell. As the Toxoplasma organisms penetrate the host cell, a parasitophorous vacuole forms from the host cell membrane. The process of active penetration takes 30 seconds under laboratory conditions. The thrusting of the conoid into the cell membrane probably is accomplished by an actin-like system. The organisms enter the host cell by active invasion, whereas entry into the macrophage can be by phagocytosis or active invasion. In naive macrophages, the organisms have the ability to actively invade the macrophage, which provides a free ride and helps in the dissemination of the infection to various organs, including the eye. In immunized hosts, the organism attains the intracellular status in the macrophage by active phagocytosis. The parasite may enter the retinal pigment epithelial cell by active penetration or phagocytosis. Retinal pigment epithelial cells have the ability to support the multiplication of T. gondii. In most host cells, however, invasion appears to be the predominant means of cellular entry by the organism. Structural differences between invasion and phagocytosis can be recognized by electron-microscopic study.4 Contact between the macrophage and the conoid may augment the penetration of the protozoan into the macrophage. If the macrophage grasps the posterior end of the organism, rhoptry discharge may not occur. The absence of microfilament aggregates under the cell membrane of the macrophage during toxoplasmic penetration is strong evidence that the cellular invasion was a parasitic effected process. Several factors may interfere with the cellular penetration, whether the penetration is by phagocytosis or by active invasion. Coating the organism with immunoglobulin G (IgG) or C'3 stimulates and enhances phagocytosis. In the absence of serum, phagocytosis may proceed, but slowly. Active invasion by the parasites may be inhibited by cytochalasin D, a substance that interferes with actin filament function.5 This cytoplasmic cytoskeletal system in the Toxoplasma organism helps it to thrust forward and move in an undulating motion.
When the parasite attains intracellular habitat, it starts to multiply, leading to host cell destruction and release of live protozoa. Live organisms do not stimulate respiratory burst during phagocytosis, but heat-killed or antibody-coated organisms generate a respiratory burst by the macrophage. The parasite may retain some of the disrupted macrophage plasmalemma adherent to the surface immediately after invasion. A vacuole quickly forms around the parasite that has invaded the host cell. The parasitophore appears to be larger than the organism, with tubules connecting membrane with the plasmalemma of the parasite. Each vacuole contains one parasite, and several organisms may be seen within a single macrophage. The Toxoplasma organism has a remarkable ability to evade the respiratory burst of macrophages by remaining within the vacuole. In activated macrophages, however, the organism has difficulty surviving within the macrophage, and the parasite is killed after fusion between the cell membrane and the internal limiting membrane of the vacuole. The organism is, in general, resistant to hydrogen peroxide, but it is susceptible to oxygen intermediates generated by the xanthine oxidase system.
LIFE CYCLE OF TOXOPLASMA GONDII
The definitive hosts of T. gondii are domestic and wild cats, whereas intermediate hosts encompass a wide variety of animals, including humans. In the definitive host, the parasite has both enteroepithelial and extraintestinal cycles. In the intermediate host, it persists in the extraintestinal cycle only. When oocysts from contaminated soil are ingested by animals, the oocyst wall is digested by the gastric enzymes, and the sporozoites are released. Similarly, when animals—including cats—ingest chronically infected tissues containing bradyzoites, the cyst wall is digested, and bradyzoites are released, leading to infection. Toxoplasma organisms then invade intestinal mucosal cells and initiate the infection (enteroepithelial cycle). In the intestinal mucosa, the organisms undergo asexual reproduction (endodyogeny, endopolygeny, splitting, schizogony) and sexual reproduction (gametogony cycles), culminating in the formation of zygotes, which develop into oocysts. The oocysts are shed in the feces of the definitive host. After oocyst excretion, sporulation occurs; the mature oocysts remain infectious in moist soil for a long time if not subjected to extreme climatic conditions.2 Freezing to -20°C, heating above 66°C, and desiccation are lethal to the cyst. Simultaneous with the enteroepithelial oocyst formation in cats, bradyzoites or sporozoites may invade and disseminate widely to all host tissue through the bloodstream or lymphatics, where they undergo an asexual cycle (extraintestinal cycle), particularly in muscle, heart, brain, lung, lymphoid tissue, retina, and central nervous system (CNS). The organism multiplies rapidly by endodyogeny in infected cells, forming pseudocysts containing tachyzoites. This usually leads to death and disruption of the cell, thereby liberating the tachyzoites, which enter contiguous cells in which they multiply, forming more pseudocysts. The rapidly proliferating tachyzoites are responsible for initial spread of the infection and tissue destruction (acute stage). In response to increased host immunity, tachyzoites transform into the slowly multiplying organisms, bradyzoites, which form true cysts in tissues (chronic stage). These can lie dormant in tissues throughout the life of the host but may cause reactivation of the clinical disease when the host's immunity is suppressed.6