HISTO SPOTS
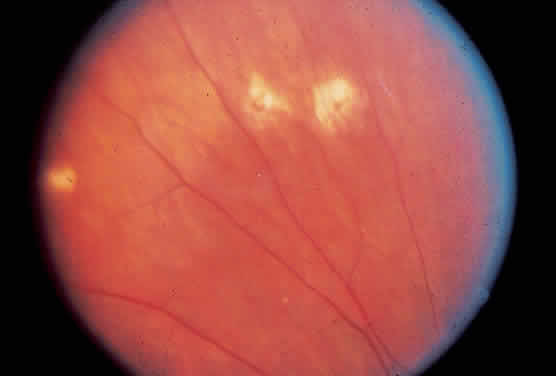
Histo spots (Fig. 1) represent a multifocal or a disseminated choroiditis characterized by atrophic or yellowish white scars in the posterior pole and the periphery. In 1972, Smith and colleagues18 showed the number of disseminated scars to range from 0 to 102. They occurred bilaterally in 62% of the patients, with the majority of the scars found posterior to the equator. The random distribution of the scars throughout the midperipheral areas and posterior pole of the fundus suggests a hematogenous dissemination of H. capsulatum to the eye. Histo spots seem to have a predilection for areas of greater blood supply. Skin testing in 59% of the general population in an endemic area was positive for the histoplasmin antigen; however, only 4.4% of this group was found to have histo scars on clinical examination.19
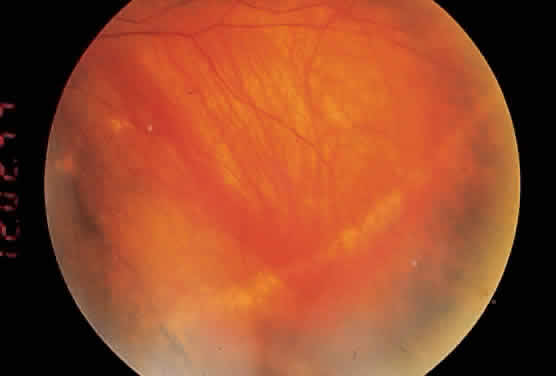
About 5% of patients with POHS demonstrate what have been called peripheral streak lesions of the fundus. These streaks are of variable length and pigmentation (Fig. 2).20 The streak lesions are most likely to be the result of a linear aggregation of histo spots. The histo spots are normally stable but may undergo changes in shape, size, and intensity. They have been shown to become larger over the course of time. The mechanism of change in size and shape of these lesions is unknown. Long-term follow-up has shown that 9% to 16% of patients will develop new histo spots.21–23 It has also been shown that 50% of eyes will have changes in atrophic scarring manifested by increased size or by formation of new scars in the peripapillary, macular, and peripheral regions. In 1969, Krill and associates24 described the evolution of these choroidal infiltrates as being hypofluorescent in the early stages of the disease. During the course of time, they assume a hyperfluorescent appearance, which is more characteristic of the mature lesions. Therefore, the late atrophic histo spot has the fluorescein angiographic picture of a pigment epithelial window defect.
|
PERIPAPILLARY ATROPHY
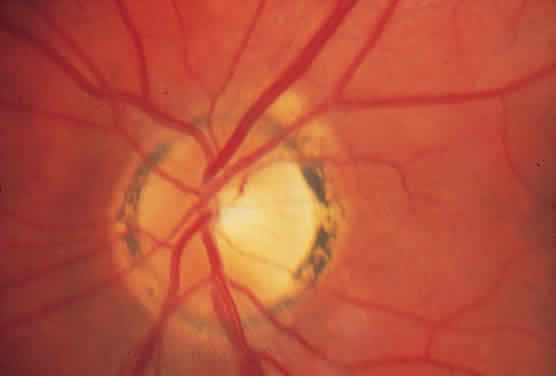
A ring of peripapillary atrophy (Fig. 3) occurs in the majority of cases of POHS. This ring is characteristic, with a narrow inner pigment zone adjacent to the disc edge and a white depigmented zone away from the disc. It is hypothesized that a circle of granulomas forms during the active infective stage of the disease and that these granulomas atrophy, leaving the ring of peripapillary atrophy. Neovascular membranes can occur in these rings of atrophy and sometimes involve the macula.25
|
MACULAR LESIONS
It is the macular lesions that bring patients to an ophthalmologist with symptoms of metamorphopsia. Subsequently, patients may develop profound loss of central visual acuity.
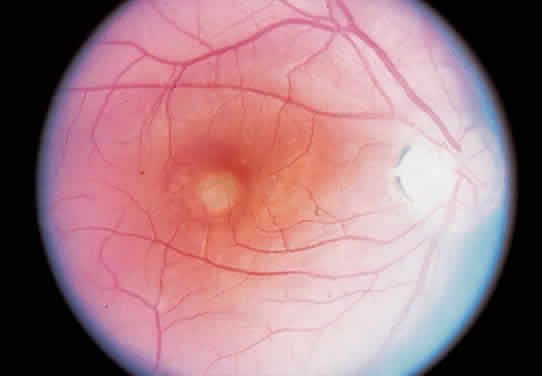
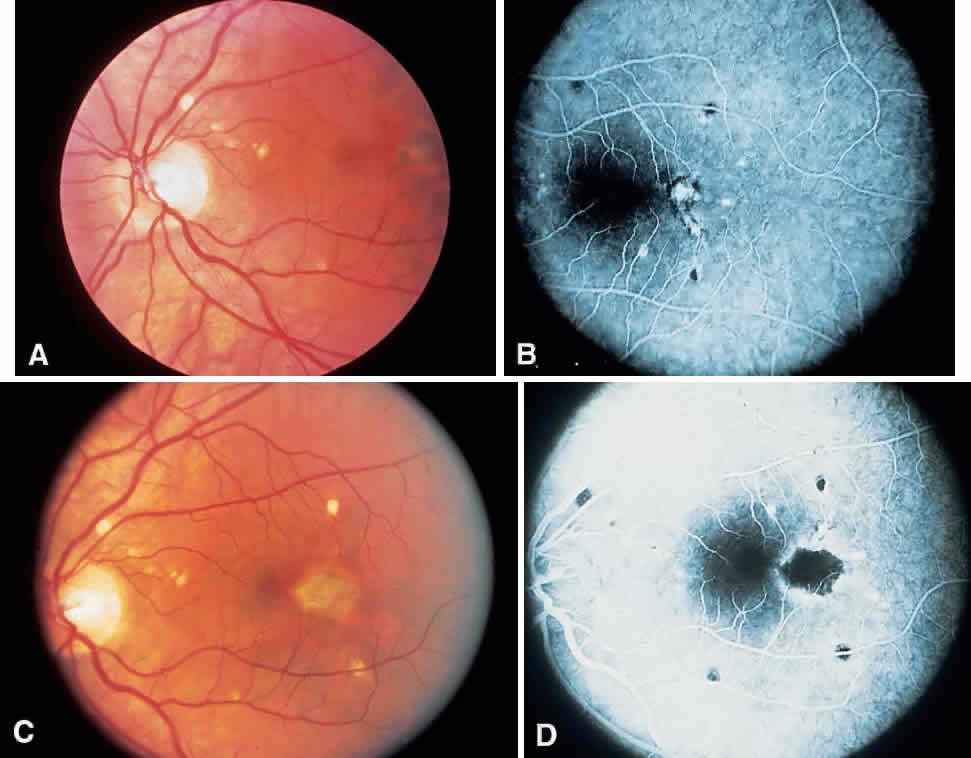
Histo spots located in the macular area tendto develop subretinal neovascular membranes (SRNVMs) (Fig. 4). These SRNVMs are similar to those seen with age-related macular degeneration, angioid streaks, and choroidal ruptures.26 Histologically, there is an egress of capillaries from the choroid to the subretinal space between Bruch's membrane and the neurosensory retina.
|
The reason for the formation of SRNVMs in the macular area in POHS, as in age-related macular degeneration, is not understood. Some investigators have postulated that an immune reaction against H. capsulatum may play a part. Others believe that the SRNVMs are just a deterioration that accompanies any break in Bruch's membrane.26 Ophthalmoscopically, SRNVMs may first appear as dirty gray-green subretinal lesions. This formation has been likened to a signet ring. The initial lesions may pro-gress to actual subretinal hemorrhage. Deposition of lipid and serous elevation of the retina may also occur as a result of increased permeability in the SRNVMs. In time, this condition progresses to an atrophic or gliotic scar, which may assume various forms. Most of the scars are white because of fibrous tissue that develops secondary to the hemorrhage. Microcystic degeneration of the sensory retina may also occur as a result of chronic serous detachment of the neurosensory retina. The visual acuity in patients who have had SRNVMs is seldom better than 20/200, unless the location of the scars is eccentric to the fovea. In one retrospective study, only 14% of untreated eyes with subfoveal neovascular networks ended with a 20/40 acuity.27 If the scar is greater than several disc diameters, the vision may fall to the counting fingers level. Just as in age-related macular degeneration, choroidal neovascular membranes may progress to massive vitreous hemorrhage. This infrequent circumstance is due to an actual hemorrhage through a break in the neurosensory retina.28,29
OCULAR MEDIA
The ocular media in patients with POHS are absolutely clear of cells. No inflammatory cells can be found in either the aqueous or vitreous. Part of the reason for this lack of cells may be because the inflammatory stage has ended by the time patients are seen clinically. Another possibility is that inflammatory cells deep in the choroid have difficulty penetrating into the vitreous. This finding is in contrast to toxoplasmosis, a retinochoroiditis, in which the primary inflammation lies in the retina. Subsequently, in toxoplasmosis, cells spill over into the vitreous and aqueous. Patients who have vitreous or aqueous cells and present with peripheral punched-out lesions, peripapillary atrophy, and macular SRNVMs should be diagnosed as having multifocal choroiditis (also called pseudohistoplasmosis).30,31 The cause of the pseudohistoplasmosis syndrome has been described to include presumed sarcoidosis, possibly tuberculosis or syphilis. In about 30% of patients, no cause of multifocal choroiditis can be determined.