EARLY BACTERIAL ENDOPHTHALMITIS
Despite the low frequency with which this entity is encountered, bacterial endophthalmitis remains one of the most dreaded and destructive complications following intraocular surgery. The combination of insults from the invading bacteria and the patient's own immune system may produce significant destruction leaving the eye with a poor visual prognosis. Prompt diagnosis and treatment are essential for achieving improved visual and functional potential. A key factor to early diagnosis is a high suspicion of endophthalmitis in postoperative cases with greater inflammation or pain than expected.
Epidemiology
The incidence of bacterial endophthalmitis has decreased significantly over the last century; unfortunately, it has not been reduced to zero. In the late 1800s, the incidence of bacterial endophthalmitis was reported to be approximately 10%.10 By the early to mid 1900s, the incidence had dropped to 0.58%11–17 and by the mid 1980s, such incidence had been reduced even further to 0.074%.18 One series published by Kattan and associates in 1990 reviewed 30,003 surgical cases performed at a single teaching hospital between 1984 and 1989.15 They found the incidence of culture-proven acute postoperative endophthalmitis to be 0.072% following extracapsular cataract extraction. Fisch and coworkers reported the incidence of culture proven postoperative endophthalmitis to be 0.31% following all penetrating ocular surgery and 0.32% after cataract surgery.19 In 1991, Javitt and partners reported the results of postoperative endophthalmitis in a nationally representative sample by reviewing all U.S. Medicare beneficiaries undergoing cataract extraction in 1984.20 Among the 338,141 patients analyzed, the risk of endophthalmitis within one year of surgery was 0.17% for intracapsular cataract extraction (ICCE) and 0.12% for extracapsular cataract extraction (ECCE). In 1996, Norregaard and associates reported similar results from a cross national comparison using data from the National Danish administrative hospital register.21 Due to the nature of broad national record collection in these studies, however; not all cases were verified by culture. In 1996, Aaberg and coworkers reported the results of a 10-year review of the incidence of culture-proven acute-onset endophthalmitis.18 They found overall incidence during this period to be 0.093%. As expected, incidence varied by surgical type. Cataract surgery with or without IOL placement had an incidence of 0.082%, pars plana vitrectomy (PPV) 0.046%, penetrating keratoplasty 0.178%, secondary IOL 0.366% and combined cataract extraction and penetrating keratoplasty 0.194%.1
Most studies evaluating the incidence of postoperative endophthalmitis have looked at patients undergoing surgery as inpatients or at major teaching hospitals. As more surgery is being performed at outpatient surgery centers, studies comparing the rates of endophthalmitis in the outpatient versus inpatient setting are of paramount importance. By 1984, about half of all cataract surgery performed on Medicare recipients was completed in an outpatient care setting.22 Between 1983 and 1984, Medicare implemented policy requiring that its recipients undergo outpatient cataract surgery except in special instances. In 1992, Javitt and coworkers published data examining the rates of postoperative endophthalmitis encountered in an outpatient versus inpatient setting.22 By comparing results from their survey of Medicare recipients undergoing cataract surgery in 1984 (before changes in Medicare reimbursement) to a cohort of patients undergoingsurgery between 1986 and 1987, they found a statistically significant decrease in the rate of endophthalmitis. At 1-month postoperative, the risk of endophthalmitis was 0.055% in the group undergoing outpatient surgery compared with 0.085% in the inpatient group. At 1 year, the risks were 0.081% and 0.12%, respectively.22 The two cohorts of patients evaluated in the study, however, were not entirely comparable. Antibiotic prophylaxis and operative technique including the proportion of cases done by small incision versus large incision surgery were not specified and may limit the clinical significance of the results.
Risk Factors
What causes some patients to develop endophthalmitis is unclear. Factors such as inoculum size, virulence of bacterial strain, intraoperative complications, and deficits in local or systemic host defenses likely play a role.23
There is significant evidence supporting the theory that bacterial entry through the wound site during surgery is the source for organisms that produce infectious postoperative endophthalmitis.24,25 In 1989, Sherwood and coworkers cultured fluid aspirated from the anterior chamber and drained from the conjunctival sac during cataract surgery in 101 patients.24 They demonstrated bacterial growth by culture of aspirate fluid from 29 patients and from the conjunctival sac of 90 patients.24 In addition, in all patients, conjunctival fluid stained with fluorescein flowed into the anterior chamber during irrigation and aspiration performed during extracapsular cataract surgery. They demonstrated that this fluid is contaminated with potentially pathogenic organisms, yet none of the patients in their study developed endophthalmitis.
Menikoff and associates conducted a case-control study of 24,105 patients undergoing intraocular surgery between 1988 and 1990 to identify risk factors for postoperative endophthalmitis.6 One factor identified as a significant independent risk for the development of endophthalmitis was intraoperative communication with the vitreous cavity.6 This complication was associated with a risk ratio of 13:7.6 Likewise, in their study of Medicare beneficiaries undergoing cataract extraction, Javitt and colleagues found that surgery accompanied by anterior vitrectomy was associated with more than a fourfold increase in the development of postoperative endophthalmitis at 1 month compared with patients undergoing cataract surgery alone.20 Several other studies have also found a higher incidence of endophthalmitis after vitreous communication has been introduced.7,21,23,26,27
Vitreous communication seems to be important in the development of postoperative endophthalmitis for several reasons. Removal of potentially pathogenic bacteria from the vitreous cavity appears to be less efficient than from the anterior chamber.9,23,28 Vitreous cultures have been demonstrated to yield a significantly higher percentage of confirmed growth and higher colony counts compared with aqueous.15,29–31 It has been suggested that vitreous may be more supportive of bacterial growth than aqueous fluid and that the viscous properties of vitreous may retard bacterial clearance.7,23,28,31
ICCE has also been associated with a higher risk of postoperative endophthalmitis compared with findings involving ECCE.20,21 Javitt and coworkers found a 0.11% risk for endophthalmitis 1 month after ICCE compared with a 0.085% risk following ECCE or phacoemulsification.20 This factor may be related to the vitreous communication allowed with intracapsular surgery.
Certain types of IOLs may also be associated with an increased risk of infectious endophthalmitis. Sherwood and associates showed that fluid bathing the conjunctiva during surgery is contaminated with potentially virulent bacteria.24 IOLs often touch various aspects of the ocular adnexa during lens insertion. Vafidis and coworkers demonstrated that factors such as electrostatic charges may cause bacteria to adhere to IOLs,32 which allows bacteria to be carried into the eye with the lens or to adhere after IOL insertion when contaminated irrigation fluid flows through the anterior chamber.24 Several investigators have shown that bacteria adhere preferentially to lenses with haptics constructed of polypropylene compared with polymethylmethacrylate.6,33 Similarly, transscleral fixation of posterior chamber IOLs with exposed polypropylene sutures has also been associated with an increased risk of infectious endophthalmitis.6
Numerous other factors have also been identified as increasing the risk of postoperative endophthalmitis. Increased intraoperative instrumentation and duration of the operative procedure seem to place patients at increased risk.6,7,9 This finding may be partly attributable to complicated cases that require anterior vitrectomy, a factor that is independently associated with an increased risk of infection. Menikoff and colleagues found patients with a history of drug allergy to be at significantly increased risk to develop endophthalmitis.6 The authors hypothesized that these patients may have a form of atopy, which is often associated with denser bacterial colonization of the adnexa and an altered immune response. Several studies have implicated diabetes mellitus with a greater risk of infectious endophthalmitis.15,19,26,34 This finding may be secondary to an impaired immune system in many of these patients. Similarly, advanced age has been associated with a greater risk of endophthalmitis. As in patients with diabetes, this finding may be explained by a relative diminution in host defenses.21
Clinical Features
A key factor in the prognosis of early postoperative endophthalmitis is prompt diagnosis and treatment. Although the ophthalmologist rarely encounters this entity, it must be kept in mind in the differential diagnosis of a postoperative patient with excessive inflammation or pain. Early postoperative endophthalmitis usually presents within the first few days after surgery. In their 10-year review of the incidence and outcomes of cataract surgery at a single institution, Aaberg and associates reported a range of 1 to 40 days, with a median of 5 days, from the initial procedure to the development of acute postoperative endophthalmitis.18 Kattan and coworkers reported similar findings in 1990, with a range from 1 to 40 days and an average of 8 days.15 Results from the Endophthalmitis Vitrectomy study, a multicentered randomized prospective clinical trial of 420 patients at 24 centers with clinical evidence of postoperative endophthalmitis following cataract extraction or secondary IOL implantation, reported a median time of 6 days until patient presentation to a study center.34 Of these patients, 24% presented within 3 days of the initiating procedure, 37% within 4 to 7 days, 17% within 8 to 13 days, and the remaining 22% presented within 2 to 6 weeks.34
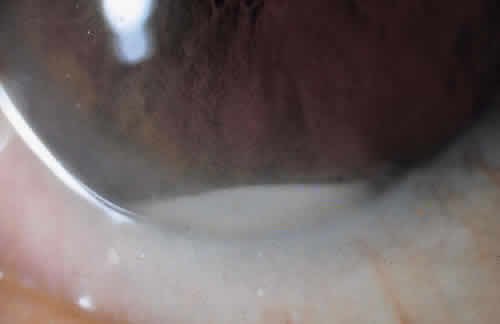
Common findings include pain, conjunctival injection, lid edema, and decreased vision. Signs and symptoms are variable, however, and they often depend on the virulence of the underlying organism.23 In the Endophthalmitis Vitrectomy study, blurred vision was the most common symptom.34 In that study, 86% of patients presented with a visual acuity of less than 5/200. Pain was reported in 74% of patients.34 Other signs include hypopyon, inflammatory pupillary membrane, vitreous opacification and inflammation, anterior chamber cell and flare, and a diminished red reflex23,34,35 (Fig. 1). Less common findings may include an afferent papillary defect and corneal ulcer or infiltrate.34 Again, these findings vary and likely depend on the underlying microorganism as well as the individual's immune response.
|
Etiology
It is generally agreed that the greatest source of bacteria causing postoperative endophthalmitis originates from the patient's own eyelid and adnexal flora.15,19,36–38 The ocular adnexa has been shown to be densely colonized with organisms commonly implicated in the etiology of postoperative endophthalmitis.36,39–41 Ariyasu and associates demonstrated that organisms cultured intraoperatively at the time of wound closure had identical typing and antibiotics sensitivities to organisms isolated from the eyelids and conjunctiva.38 By using restriction enzyme endonuclease studies, Speaker and partners found that 82% of organisms isolated from the vitreous of patients with postoperative endophthalmitis were genetically indistinguishable from isolates recovered from the patient's eyelid, conjunctiva, and nose.37
Gram-positive, coagulase-negative micrococci, including Staphylococcus epidermidis are responsible for most cases of postoperative endophthalmitis.15,18,19,23,26,34,38,42 Kattan and coworkers reported that 78% of organisms cultured following cataract surgery between 1984 and 1989 consisted of gram-positive species.15 More cases were attributed to S. epidermidis than to any other species. In their group of all patients with culture-positive endophthalmitis from 1990 to 1994, Aaberg and coworkers found 76.7% of patients grew gram-positive organisms, 47.8% of which were S. epidermidis.18 In the Endophthalmitis Vitrectomy study (EVS), gram-positive, coagulase-negative micrococci represented 70% of the 323 isolates meeting the study criteria for confirmed growth.42 This group consisted almost entirely of S. epidermidis with a single isolate of Micrococcus kristinae.42 The next most common group consisted of other gram-positive species, including S. aureus (9.9%), 29 isolates of Streptococcus species (9.0%), and 7 isolates of Enterococcus species (2.2%). The least common group of organisms consisted of gram-negative species, representing only 5.9% of isolates.42 Polymicrobial growth occurred in 9.3% of the EVS patients. In this group, two species of gram-positive, coagulase-negative micrococci grew in more than half of patients.42 It is clear that organisms typically found colonizing the ocular adnexa are not so benign as once believed.40,43 They appear to play a significant role in the pathogenesis of postoperative endophthalmitis.
Diagnosis
One of the most important aspects in the prognosis of postoperative endophthalmitis is the physician's willingness to make a diagnosis. This requires maintaining a high level of suspicion for patients who are at greater risk preoperatively and for patients who have greater postoperative inflammation than expected. If postoperative inflammation is disregarded and other signs and symptoms of endophthalmitis go unnoticed, critical time may be lost and prompt diagnosis be delayed.
In the EVS, 24% of patients presented within 3 days of the surgical procedure; 61% presented within 1 week.34 These figures emphasize the importance of early and frequent postoperative examinations, especially in complicated surgical cases. Patients should be educated regarding the normal postoperative course and counseled about warning signs such as excessive pain and decreased vision. Patients should also be instructed to call their physicians promptly if these symptoms begin.
In cases of suspected endophthalmitis, a careful history and examination are of utmost importance. Systemic diseases such as diabetes mellitus, which might put the patient at increased risk for the development of endophthalmitis or might point to a source of endogenous infection, should be noted. Factors such as antibiotic and steroid use and systemic immunosuppression should also be determined. Details regarding the operative course such as duration of the case, presence of capsular rupture, or vitreous loss are important.6,44 Physical examination should include a careful determination of visual acuity as this influences the immediate course of treatment.34 The pupils should be examined and any afferent pupillary defect noted. Findings such as the degree of anterior chamber inflammation, the presence of a hypopyon or corneal infiltrate, and clarity of the vitreous should be noted. Loss of vision, vitreous opacification, and the presence of a hypopyon are some of the findings consistent with acute postoperative infection.23 In general, if a hypopyon is present, one needs to assume that the source is infectious until proven otherwise. Integrity of the wound should be checked by looking for signs of wound leak, vitreous wicks, and the presence of any suture material that might provide an infectious tract. The conjunctiva should be checked for evidence of a bleb and the iris examined for rubeosis. IOL position, stability, and capsular integrity should be noted. The capsule should also be examined for evidence of white plaques, which may suggest an infectious process especially in late postoperative endophthalmitis. Retained lenticular material should also be noted as this can cause inflammation, which may mimic infectious endophthalmitis. This is especially important if the posterior capsule is no longer intact. Ultrasound can be useful in the diagnosis of retained nuclear material, which may not be visualized on clinical examination. Examination of the posterior segment should include noting the presence or absence of a red reflex, degree of retinal vascular visualization, retinal detachment, choroidal detachment, or vitreous membrane. Retinal detachments are difficult to repair in patients with underlying endophthalmitis and usually confer a poor visual prognosis.35 When the posterior segment cannot be properly visualized, B-scan ultrasound should be performed.
After endophthalmitis has been suspected and a thorough history and physical examination completed, diagnosis is confirmed by completion of appropriate cultures. External cultures of the eyelid margins and conjunctiva may be obtained. Both aqueous and vitreous cultures should be obtained before injection of any antibiotics or steroids. A vitreous sample is especially important because a large number of aqueous samples demonstrate negative growth. In the EVS, confirmed growth on culture was demonstrated from 54.9% of vitreous samples compared with only 22.5% of aqueous samples.31 In cases in which a vitreous sample was positive, Bode and colleagues demonstrated negative growth in up to 57% of aqueous cultures.30 Given these findings, a vitreous sample is absolutely imperative for adequate diagnosis.
Vitreous specimens can be obtained either by trans-pars plana needle aspiration or by vitreous biopsy with a vitrectomy unit. In the EVS, vitreous was cultured by one of three methods: needle aspiration, vitreous biopsy with the vitrector, or vitreous biopsy followed by vitrectomy (vitreous effluent was also cultured in these cases). No statistically significant difference was found in the yield among the three groups.31 Some authors have suggested that a vitreous culture using a vitrector unit may be superior because a higher inoculum is obtained, and a larger specimen is collected.45 This may aid in culture positivity and assist in clinical recovery by removing a greater number of infectious organisms from the eye. In addition, aspiration of vitreous with a syringe is often difficult and can place traction on the retina.23 If needle aspiration is attempted and fails, a vitreous biopsy using a vitrectomy unit should be performed.
When a vitrectomy is used for biopsy and culture, an initial undiluted vitreous specimen should be obtained after placement of the sclerotomies, but before the infusion is turned on. The cutter can then be introduced in to the vitreous and a vitreous sample removed and aspirated into a syringe. Following sample collection, the infusion can be turned on.34 Vitreous effluent from the vitrectomy cassette can be collected and cultured following the surgical case. The effluent is usually processed through filter paper as in the EVS.31 The dilute effluent can also be centrifuged to produce a concentrated specimen.23
After specimens have been collected, samples are cultured on appropriate media. These include blood, chocolate, thioglycolate broth, and Sabouraud dextrose agar, all incubated at 37°C. Gram and Giemsa staining should also be performed. The presence of a positive result on Gram stain has been associated with a higher incidence of culture positivity.31 In the EVS, the positive predictability of the Gram stain was greater than 94%.31 A positive culture is often defined as at least semiconfluent growth on a solid medium, any growth on two or more media, or growth on one medium with a positive Gram stain.34,46 Detection of bacterial DNA using polymerse chain reaction (PCR) may be considered for cases in which infectious endophthalmitis is clinically suspected, but in which negative cultures have been obtained.47,48 Okhravi and associates reported a 100% concordance rate between PCR- and culture-positive bacterial samples and demonstrated that the technique is useful for detecting infection in cases which are culture negative.47
Treatment
The aims in treating patients with postoperative endophthalmitis include sterilization of the eye and control of inflammation.
Intravitreal antibiotics are the initial treatment of choice for postoperative endophthalmitis.23 Two drugs are typically used. One should have broad and effective activity against gram-positive species, specifically coagulase-negative staphylococci, and the other should protect against gram-negative bacilli. In the EVS, intravitreal vancomycin and amikacin were administered to all patients and demonstrated effective antimicrobial activity against most pathogens.34,44 Vancomycin is the traditional drug of choice for gram-positive coverage. It is the only agent tested to which all gram-positive species in the EVS were susceptible.44 Development of vancomycin-resistant strains of staphylococcal and streptococcal species has prompted the Centers for Disease Control and Prevention (CDC) to recommend reserving use of vancomycin for treatment of serious infections produced by β-lactam resistant gram-positive microorganisms.49 Cefazolin (Ancef) has activity against many gram-positive organisms. However, in a rabbit model of S. aureus endophthalmitis, cefazolin was less effective than vancomycin and clinical resistance of gram-positive organisms is frequent.50,51 In light of the absence of data suggesting an effective alternative to vancomycin, and the potentially devastating consequences of endophthalmitis, vancomycin remains the agent of choice for the coverage of gram-positive organisms in cases of postoperative endophthalmitis. Based on susceptibility data from the EVS, vancomycin in combination with either amikacin, ceftazidime, or ciprofloxacin are appropriate antibiotic choices.44 Many practitioners have moved away from using gentamicin because it is more toxic than amikacin in experiments using rabbit eyes. Additionally, macular infarction has been reported after its administration in patients.52,53
Periocular antibiotics may be used as an adjunct to intravitreal therapy.34 Subconjunctivally injected antibiotics have poor intravitreal penetration54 but can achieve high concentrations in the anterior segment. Similarly, topical antibiotics provide poor intravitreal penetration but can achieve high concentration in the anterior segment and help sterilize wound sites.44 It is still unclear whether subconjunctival and topical antibiotics offer additional benefit in the treatment of postoperative endophthalmitis.
The role of corticosteroids in the treatment of postoperative endophthalmitis is still somewhat controversial. In the EVS, corticosteroids were administered to all patients subconjunctivally, topically, and orally, although the study was not designed to determine the efficacy of this adjunctive therapy. Corticosteroids play an important role in controlling the inflammatory response after infection, which may be further damaging to the eye. Corticosteroid use intravitreally may help control intraocular inflammation, especially after bacterial death from antimicrobials. Unless fungal endophthalmitis is suspected, some practitioners recommend the use of intravitreal steroids in conjunction with intravitreal antibiotics.23,55 Several studies have suggested that administering intravitreal corticosteroids may be beneficial,56–58 although at least one study found that intraocular steroid use was not associated with a significant effect on visual acuity.18 No prospective, controlled clinical trials have yet proved the efficacy of intravitreal corticosteroids in the management of postoperative endophthalmitis.
The EVS determined that patients who presented with initial vision of only light perception who underwent a vitrectomy had a three times greater chance of achieving 20/40 final visual acuity (33% versus 11%) compared with patients who had only a vitreous tap or biopsy. There was no difference in outcome between vitrectomy and vitreous tap in patients with endophthalmitis who presented with initial visual acuity of hand motions or better.34
Before the results of the EVS, systemic antibiotic use was considered a standard treatment for postoperative infectious endophthalmitis.34 The EVS concluded, however, that there was no difference in visual acuity or media clarity outcome with or without the use of systemic antibiotics.34 These results were important for several reasons. Systemic antibiotics may have significant adverse effects and their use is not without risk. Systemic antibiotics are also expensive. Not only do the drugs incur expense, they usually require either admission to a hospital or home-based nursing for their administration. Thus, the findings of the EVS may save the patient unnecessary risk and may obviate the need for hospital admission. However, results of the EVS may need to be reevaluated in light of new fluoroquinolone antibiotics that have better intraocular penetration compared with that of other antibiotics.9,59
Of utmost importance in the treatment of postoperative endophthalmitis is prompt initiation of therapy. This point cannot be overemphasized. Endophthalmitis is an ocular emergency and initiation of treatment should reflect this. The visual outcome of the patient is strongly connected to the efficiency with which a diagnosis of endophthalmitis is made. In addition, the results of the EVS and other studies have provided significant guidelines for the treatment of postoperative infection, but one must keep in mind that each case must be evaluated on an individual basis. The patient must be monitored closely and decisions to change treatment strategies or initiate a surgical procedure must be considered at each step.
Prognosis
Despite the fear provoked by a diagnosis of postoperative endophthalmitis, final visual acuity can be surprisingly good. In general, the worse the acuity at initial presentation, the worse the final visual outcome will be.44 In the EVS, 41% of patients achieved 20/40 or better visual acuity at 3 months. By the end of the study, more than half of patients (53%) achieved visual acuity of 20/40 or better and 74% achieved 20/100 vision or better. Only 11% of patients had acuity worse than 5/200.34 Other studies have found similar results. Kattan and colleagues found that approximately one third of patients achieved a final visual acuity of 20/60 or better following endophthalmitis after cataract extraction with or without IOL placement.15 Speaker and Menikoff found 64% of patients with endophthalmitis after intraocular surgery had a visual acuity of 20/100 or better after treatment.26
Final visual acuity after postoperative endophthalmitis varies according to surgical type. Aaberg and coworkers found the median visual acuity after endophthalmitis treatment was 20/200 overall. Postcataract extraction endophthalmitis patients had a median final acuity of 20/133, and the best median visual acuity, 20/40, was obtained after secondary IOL placement. As may be expected, patients with post-PPV endophthalmitis fared worst, with a median acuity of no light perception.18
The EVS and other studies have found certain risk factors associated with a worse visual acuity outcome after treatment for postoperative endophthalmitis. According to the EVS, the most significant independent risk factor for decreased vision was visual acuity of light perception or worse at initial presentation.34 Other independent risk factors included advanced age, diabetes, presence of a corneal infiltrate and/or ring ulcer, intraoperative violation of the posterior capsule, low or high intraocular pressure, presence of an afferent pupillary defect, rubeosis, and absent red reflex.34,44 Studies have demonstrated that more virulent organisms, including S. aureus, Streptococcus species, and gram-negative rods are often associated with worse visual prognosis.19,23,60 A shorter time interval between the onset of symptoms and initiation of treatment also plays a role in visual outcome following postoperative endophthalmitis.60 In the EVS, initiation of treatment was begun within 6 hours of the initial exam.34 Visual results after treatment were better in the EVS than in many other studies.15,18,26,60 This may have resulted in part from efficient initiation of treatment, underscoring the importance of prompt diagnosis and treatment. The first few hours in the management of postoperative endophthalmitis are critical.
Prevention
Preventive measures play an important role in reducing the incidence of postoperative endophthalmitis. The practitioner can play an active role in the prevention of infection by choosing to incorporate appropriate aspects into perioperative management of his or her patients.
Preparation of the surgical field with sterile drapes does not eliminate the risk of infection, but it can reduce it.61 Adhesive drapes, which cover the eyelid margin and lashes, may be particularly important in reducing the chance of infection from microbial contaminants of the ocular adnexa.
Various topical antiseptics have been used for the preoperative preparation of the eye. Argyrol, a mild silver protein solution, was commonly used for many years. In 1983, however, Isenberg and associates demonstrated that the chemical preparation was no more effective than no treatment in reducing the number of bacteria.62 In addition, the same investigators evaluated the effect of conjunctival irrigation in the preoperative chemical preparation of the eye.63 They determined that both the bacteria colony count and species count increased following irrigation with saline solution compared with nonirrigated eyes.
Unlike many more traditional topical antiseptics, povidone-iodine has been shown to have significant antimicrobial effect.26,64–69 Povidone (polyvinylpyrrolidone) is a high-molecular-weight, water-soluble polymer that forms a complex with iodine.68 After instillation, free iodine is released, which has been credited with the compound's antimicrobial effect.68,70 Povidone-iodine possesses minimal tissue toxicity and has a broad antimicrobial effect with activity against bacteria, viruses, fungi, protozoa, and spores.67,68 Maumenee and Michler first studied the use of iodine solution in ophthalmic surgery in the early 1950s.71 They found it possessed an antimicrobial effect on the ocular adnexa when the solution was used concomitantly on the skin. In 1984, Apt and coworkers demonstrated that eyes treated with half-strength povidone-iodine solution preoperatively had a 91% decrease in bacteria colony counts and a 50% decrease in the number of species compared with those found in controls.68 The solution did not cause significant conjunctival hyperemia and no allergic or adverse reactions were reported. Speaker and Menikoff demonstrated that the preoperative application of 5% povidone iodine to the ocular surface significantly lowered the incidence of culture-positive endophthalmitis after intraocular surgery compared with eyes prepared with a silver protein solution.26 Among more than 3000 cases in which povidone-iodine was used, the solution was well tolerated and no adverse reactions were noted.26
In most studies of povidone-iodine, a 5% solution is prepared by diluting full-strength 10% solution 1:1 with saline solution.23,26,68 Two drops of the solution are instilled in the conjunctival sac, and the eyelids are gently manipulated to distribute the solution over the surface of the eye. The solution bathes the eye while the rest of the preparation is completed. The solution should remain in contact with the eye for several minutes to exert its antimicrobial effect. The skin, eyelid margins and lashes are then scrubbed with povidone-iodine-soaked gauze or cotton swabs. Finally, the eye is irrigated with saline solution at the end of the preoperative preparation. This step is important to avoid intraocular toxicity with the solution.26 Povidone-iodine solution should not be confused with povidone-iodine scrub. The latter contains a detergent, which is toxic to the eye and should be avoided.26
Use of perioperative antibiotics in ophthalmic surgery remains controversial. Short-course topical antibiotics administered before surgery reduce the amount of lid and conjunctival bacteria.69,72,73 The amount of pathogen reduction depends on the type of antibiotic used, the frequency and duration of its use, and the type of bacteria present and the susceptibilities of the organism.73
Inasmuch as the major infectious source for postoperative endophthalmitis is the patient's own eyelids and conjunctiva.9, it is reasonable to attempt to reduce the number of organisms with prophylactic antibiotics. Up to three quarters of patients carry organisms on the ocular adnexa capable of causing postoperative endophthalmitis.40 In 1964, Allen and Mangiaracine suggested that preoperative topical antibiotics reduced the incidence of endophthalmitis.74 At that time, chloramphenicol and gentamicin were routinely used as antibiotics. Studies using these antibiotics have shown variable efficacy.75–77 More recently, studies employing fluoroquinolone antibiotics have reported favorable results. This family of antibiotics has broad-spectrum antimicrobial activity, low rates of resistance, and minimal toxicity.78 Donnenfeld and associates demonstrated that patients given topical 0.3% ofloxacin 30 and 90 minutes preoperatively had aqueous humor drug levels that approached the minimal inhibitory concentration of 90% of Staphylococcus epidermidis, S. aureus, and most gram-negative organisms.78 Von Gunten and coworkers reported similar results.59 Snyder-Perlmutter amd and colleagues reported that patients receiving topical antibiotics every 5 minutes for three doses had a statistically significant reduction in bacterial colony-forming units following treatment with ciprofloxacin but not ofloxacin.79 These studies suggest that topically applied antibiotics might decrease the intraocular bacteria count sufficiently to prevent development of infectious endophthalmitis. The low incidence of endophthalmitis, however, makes it difficult to prove that a direct correlation exists between antibiotic use and a reduction in the incidence of infectious postoperative endophthalmitis.
Subconjunctival antibiotics have been commonly used since the 1950s for infectious prophylaxis following ocular surgery.80 During this time their use has been a source of continued controversy. Administration of subconjunctival antibiotics circumvents the corneal epithelial barrier and permits a high concentration of drug to penetrate the corneal stoma and diffuse into the aqueous humor.9 Studies have demonstrated good penetration of antibiotics into the anterior chamber following subconjunctival administration.44,81 A subconjunctival injection of antibiotics can achieve bactericidal levels in the anterior chamber thus eliminating most infection.9 It has been suggested, however, that high intraocular levels of antibiotic achieved with subconjunctival injection might only partially treat an infection in some cases and delay onset of symptoms and diagnosis leading to a poor outcome.82 Additionally, the concentration of anterior chamber antibiotic decreases rapidly after administration and vitreous penetration following subconjunctival administration of antibiotics is also poor, limiting its efficacy.9,23,54,73
Studies evaluating the efficacy of subconjunctival antibiotics in decreasing the incidence of endophthalmitis have been inconclusive. In 1967, Cassady published an uncontrolled series of 1212 cataract extractions in which subconjunctival antibiotics were used.27 No cases of postoperative endophthalmitis were reported. Cassady attributed his success to the use of subconjunctival antibiotics and justified their usage after every cataract extraction. During that same year, a controlled, prospective study was reported evaluating subconjunctival antibiotic use following cataract surgery.83 Two cases of endophthalmitis were reported in both the control and treatment groups. Unfortunately, the small number of infections prevented any conclusions beingdrawn regarding the statistical significance of antibiotic prophylaxis. In another study, Kolker and associates reported a 1.42% incidence of postoperative endophthalmitis in patients given no prophylaxis versus 0.21% in a similar group given subconjunctival penicillin and streptomycin.84 In two series reviewing postoperative endophthalmitis, all but one patient had received postoperative subconjunctival antibiotics.15,26 No definitive evidence has elucidated the appropriate role of subconjunctival antibiotics in surgical prophylaxis. Issues of efficacy, safety, and cost must be considered and a controlled, prospective clinical trial must conducted before a final verdict is reached.
Aminoglycosides were commonly used for subconjunctival injection, but such usage has fallen into disfavor. Gentamicin has been associated with more conjunctival toxicity and greater patient discomfort than cefuroxime.85 Aqueous penetration of gentamicin is poorer than cephalosporins and more species that are implicated in the etiology of postoperative infectious endophthalmitis are sensitive to cephalosporins than aminoglycosides.85 Retinal toxicity has also been demonstrated following aminoglycoside use. In 1991, a survey of members of the Retina, Macula and Vitreous Societies revealed 93 cases of macular infarction following administration of gentamicin sulfate with eight additional cases secondary to other aminoglycosides.53 Most reports followed planned intravitreal injection of antibiotic but twenty-three cases occurred in eyes treated with prophylactic subconjunctival antibiotics after routine ocular surgery. Of these cases, most occurred following known or presumed ocular penetration; at least two cases were thought to be associated with drug leakage into the eye through the cataract wound following injection. Aminoglycosides have an extremely small therapeutic window, making their use for routine surgical prophylaxis unwise.53 If even a small amount of gentamicin dosed for subconjunctival use gains entry to the eye, retinal toxicity could occur.80 Compochiaro and Conway pointed out that endophthalmitis is treatable whereas macular infarction is not; they thus recommended that use of prophylactic subconjunctival aminoglycosides be abandoned following routine ocular surgery and subconjunctival use of other antibiotics be administered only after serious consideration and careful surgical attention.53
Intracameral antibiotic use, including antibiotic administration in irrigating solutions and through direct injection, is another method of infectious prophylaxis that has gained popularity recently. Preliminary toxicity studies have been done in animals, but difficulty has been encountered when equivalent antibiotic doses are applied in humans.86,87 This has resulted in disagreement regarding clinical doses and formulations of prophylactic intracameral antibiotics.88–90 Theoretically, intracameral antibiotics may reduce the incidence of postoperative infectious endophthalmitis, but the clinical evidence supporting their use is largely circumstantial. No prospective, controlled clinical trials have been completed to date to address this question. An uncontrolled study by Gills reported one case of endophthalmitis in 20,000 cataract extraction following gentamicin use in irrigating solution and one case in 9928 after intracameral vancomycin.88 No cases were reported following 25,000 operations in which both gentamicin and vancomycin were used in irrigating solution. In another uncontrolled study, Gimbel and partners reported no cases of endophthalmitis following 4,684 cataract extractions in which both intracameral gentamicin and vancomycin were used.90 Those authors reported no adverse effects on corneal endothelium when cases were compared to similar series following phacoemulsification with lens implantation and no intracameral antibiotics, but endothelial damage may have been spared because vancomycin instead of being infused in the irrigating solution was delivered into the capsular bag by intracameral injection after lens placement. Beigi and colleagues reported that the addition of gentamicin and vancomycin to irrigation solution during phacoemulsification significantly reduced contamination of anterior chamber aspirates with bacteria.89 No patients in the study developed postoperative endophthalmitis, however, making it impossible to determine whether intracameral antibiotic use decreased the incidence of postoperative infection. One should also note that the CDC has cautioned against the routine use of vancomycin.49
DELAYED-ONSET BACTERIAL ENDOPHTHALMITIS
Awareness of delayed onset or late endophthalmitis has increased in recent years. As with acute infectious postoperative endophthalmitis, recognition of this entity is of utmost importance in early diagnosis and improved visual prognosis. The signs and symptoms of delayed onset endophthalmitis can be subtle, often mimicking other causes of inflammation and making diagnosis difficult.
Definition
Many terms have been used to describe this entity including delayed-onset, chronic, and late postoperative endophthalmitis. A consensus regarding the time period in which this disorder occurs has also been disputed. Some authors have suggested that symptoms starting more than 1 month postoperatively should be considered chronic endophthalmitis, whereas other authors have defined chronic endophthalmitis as requiring two or more episodes of low-grade postoperative infectious uveitis regardless of the time of onset.91–93 The Endophthalmitis Vitrectomy Study assessed acute postoperative endophthalmitis and evaluated patients with evidence of endophthalmitis within six weeks after surgery.34 For our purposes, we will define delayed-onset postoperative infectious endophthalmitis as uveitis that develops more than 6 weeks after surgery with evidence of an infectious process. Even so, this definition is somewhat arbitrary and merely serves as a guideline for classification purposes.
Epidemiology
Most data estimate incidence of acute postoperative infectious endophthalmitis or do not distinguish between acute and chronic infections when reporting statistics of incidence.15,18,19,21,91 Unfortunately, this makes it difficult to assess the frequency with which delayed-onset endophthalmitis is encountered. In addition, studies have evaluated risk factors associated with acute postoperative endophthalmitis, but data are lacking with regard to infection of delayed onset.6,91
Etiology
Propionibacterium acnes was the first bacterial organism reported to cause chronic low-grade inflammation after intraocular surgery.91,94 In 1986, Meisler characterized a new syndrome of delayed onset endophthalmitis and reported six cases of chronic postoperative endophthalmitis masquerading as chronic iridocyclitis from which Propionibacterium species were cultured.95 Other bacteria can cause a similar picture of chronic low-grade inflammation, including coagulase-negative Staphylococcus,96–98 Staphylococcus aureus,99,100 anaerobic Streptococcus,101 Corynebacterium species,96 Actinomyces,102,103 and Nocardia species.104 Various fungi, particularly Candida species, can produce a similar picture.96,105,106 Despite the array of organisms implicated in delayed-onset postoperative endophthalmitis, P. acnes is most often encountered.
P. acnes is a gram-positive, non-spore-forming anaerobic pleomorphic bacillus that has been implicated in a wide variety of systemic infections.92 It is a ubiquitous organism, which inhabits the human sebaceous follicle.102 It has been cultured in up to 43.8% of normal human conjunctivae.43 In addition to chronic endophthalmitis, ocular and periocular infections from P. acnes have included preseptal cellulitis, canaliculitis, dacryocystitis, conjunctivitis, and keratitis.107 Several cases of P. acnes-induced acute postoperative endophthalmitis have also been described.93,102
P. acnes cell wall resists digestion by macrophagesand polymorphonuclear cells, which allows it to persist sequestered in inflammatory cells within the eye.91,101,108 Some evidence has suggested that P. acnes can also live within the lens capsule, protected from inflammatory cells and attempts to sterilize the eye with antibiotics.109 Release of sequestered organisms may explain the onset of endophthalmitis that has been reported after treatment with capsulotomy using neodymium/yttrium-aluminum-garnet (Nd:YAG) lasers.95,109,110
Clinical Features
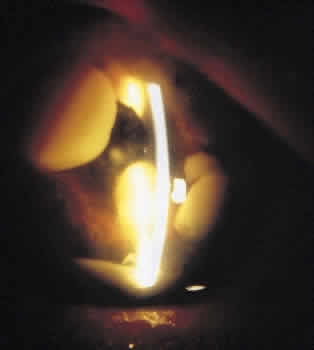
Patients with delayed-onset postoperative endophthalmitis may present with signs and symptoms typically found in patients with acute endophthalmitis. Most often, however, they present with low-grade chronic and often recurrent inflammation that may simulate noninfectious uveitis. Such inflammation occurs over the course of months compared with the abrupt inflammatory presentation seen in acute postoperative infections. Patients may initially show some improvement following corticosteroid treatment, which hinders the correct diagnosis of endophthalmitis. Another finding that separates this entity from acute endophthalmitis is development of granulomatous precipitates on the corneal endothelium and IOL.92,95,110,111 The finding of white plaques on the posterior capsule is also suggestive, although not pathognomonic for P. acnes infectious postoperative endophthalmitis.91,92,101,112,113 A similar plaque has also been described in other causes of bacterial and fungal endophthalmitis.114,115 The plaque is usually located between the IOL and the posterior capsule or peripherally in the capsule, requiring adequate dilation for visualization.92,101 (Fig. 2). Histologically, the plaque consists of a mixture of lens material and colonies of microorganisms.92,101 Other clinical findings may include hypopyon, a fibrinous reaction in the anterior chamber manifested as beaded fibrin strands, as well as vitritis and cystoid macular edema.95,102,109,110 Patients usually develop symptoms between 1 and 10 months after surgery, although the time elapsed to culture proven infection is often delayed owing to initial misdiagnosis.95,96,102,109,110,116
|
Diagnosis
As in cases of acute postoperative endophthalmitis, aqueous and vitreous cultures should be obtained. Owens and coworkers suggest directing the anterior chamber tap needle into the capsular bag or toward the plaque to increase pathogen yield.117 Likewise, it is desirable to acquire a section of the capsule or plaque when attempting to obtain a vitreous specimen.91,92 This should be sent for culture as well as histopathologic studies.92,110 Specimens should then be sent for both Gram stain and culture, including culture on anaerobic media. The specimens must be incubated for at least 1 to 2 weeks because it may take longer than expected to observe growth.92 As in cases of acute infectious endophthalmitis, PCR may be helpful in establishing a diagnosis.47,48
Treatment
No consensus exists regarding the best treatment for patients with chronic infectious postoperative endophthalmitis. Various treatment strategies have been suggested but the individual patient must be kept in mind when deciding on a therapeutic plan.
Some authors have suggested that initial treatment should consist of medical therapy such as topical and systemic antibiotics alone, or in combination with intraocular antibiotics.93,102,111 P. acnes has been shown to be sensitive to penicillins and cephalosporins, but vancomycin may be the intraocular antibiotic of choice.91,92,111 Vancomycin is not only effective against P. acnes, but provides coverage against other bacteria such as coagulase-negative streptococcus, which can cause delayed-onset endophthalmitis.51,91 Other antibiotics such as clindamycin and chloramphenicol can also be effective.91,117 Zambrano and coworkers reported successful treatment using a nonsurgical approach consisting of topical, intraocular and/or intravenous antibiotics in four of nine cases reviewed at Bascom Palmer Eye Institute.102 Clindamycin irrigation into the capsular bag has also been reported to be useful in treating P. acnes delayed-onset endophthalmitis,117,118 although another series reported recurrent inflammation in 100% of patients receiving intraocular antibiotic treatment alone.111
Most cases will go on to require some type of surgical treatment due to recurrent or persistent inflammation. Zambrano and colleagues proposed an algorithm to treat patients with delayed-onset infectious postoperative endophthalmitis.102 They suggested that patients who present with mild symptoms on initial examination have cultures performed and be given intraocular vancomycin. If that therapy is ineffective, those authors suggest that PPV with excision of the intracapsular plaque and repeat antibiotic injection be completed while maintaining sufficient capsule to support the IOL. Patients presenting with more severe clinical findings may receive this treatment initially. Finally, if this approach does not alleviate inflammation, IOL removal with complete capsular bag removal and possible IOL exchange can be performed.102 Clark and colleagues111 found that patients treated with intra-ocular antibiotics alone or a combined therapy with PPV and intraocular antibiotics had high rates of recurrent and persistent inflammation (100% and 50%, respectively.) Patients receiving PPV with subtotal capsulectomy and intraocular antibiotics had a 14% incidence of inflammation following treatment. No recurrent or persistent inflammation was reported for those patients who later went on to receive PPV, total capsular bag removal, intraocular antibiotics, and either IOL exchange or removal.111
Despite various available treatment options and the lack of ideal therapeutic alternatives, the visual prognosis for these patients can be relatively good.93,102,111,119 Clark and colleagues reported final visual acuities to be 20/40 or better in 50% of patients and 20/400 or better in 75%.111 Zambrano and coworkers reported final visual acuities for two thirds of patients to be between 20/20 and 20/60 and Winward and coworkers reported final visual acuity of 20/70 or better in 70% of patients.93,102 Poor final visual acuity is often attributed to cystoid macular edema, retinal detachment, retinal vascular events, glaucoma, or phthisis bulbi.92,102,111
FUNGAL ENDOPHTHALMITIS
The first case of fungal endophthalmitis was reported by Romer in 1902 following a case of penetrating trauma with subsequent Aspergillus fumigatus infection.120 Fungal endophthalmitis is usually considered in the differential diagnosis of indolent postoperative inflammation, although reports of acute postoperative fungal endophthalmitis have also been cited.105,106,121 Fungal infections are relatively rare and can resemble bacterial endophthalmitis, making diagnosis difficult.105,114 When this diagnosis is being entertained, diagnostic measures should be completed expeditiously because a delayed diagnosis may be associated with a poorer visual prognosis.106
Clinical Features
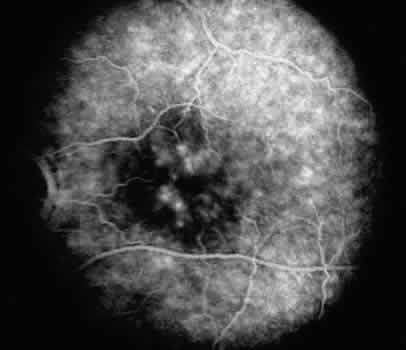
Some clinical signs may help distinguish fungal endophthalmitis from other sources of infection. Fungal infections may exhibit “fluff balls” or a “string-of-pearls” infiltrate in the anterior chamber and vitreous.91,101,122,123 The infection tends to be more localized with inflammation often limited to the anterior chamber, pupillary space, or anterior vitreous,105,123 and a mass may be seen on the iris, ciliary body, lens, or adherent to the cornea105,106,124 (Fig. 3). Reports have demonstrated that fungus can infiltrate these ocular tissues, which serve as a reservoir for persistent infection.105 Other findings can include persistent corneal edema, corneal infiltrates or necrotizing scleritis.91,110,111,125 As in chronic bacterial endophthalmitis, a hypopyon or white plaque may be seen, and low-grade persistent and recurrent inflammation is often noted.91,101,106,121,122
Various organisms have been reported to cause postoperative fungal endophthalmitis. These include Candida parapsilosis, Aspergillus flavus, Torulopsis candida, Torulopsis magnoliae, Paecilomyces lilacinus, Acremonium species,105,106,114,122,126,127 and others. Exogenous fungal endophthalmitis typically occurs following cataract surgery, but reports have also documented infection after penetrating keratoplasty, retinal surgery, and glaucoma filtering.24,80,106,128 Pflugfelder and coworkers noted that some fungi appear to be associated with a better visual prognosis including Aspergillus, Paecilomyces, and Candida, whereas infections secondary to Acremonium and Fusarium species typically have poorer outcomes.105
Treatment
When a fungal infection is suspected, cultures should be obtained expeditiously. Given the variability of clinical presentation and the possibility of fungal infection mimicking bacterial endophthalmitis, it is reasonable to culture intraocular specimens for both bacteria and fungi in all patients with suspected endophthalmitis.105 Cultures should be obtained in the same fashion outlined for bacterial endophthalmitis. When a capsular plaque is present, this should be harvested for histologic examination. Similarly, aspiration or excision of any mass that may be present in the anterior chamber, or adherent to the cornea, iris, or lens is important for both diagnosis and treatment.105 Both Giemsa and Gomori methamine silver stains should be obtained in addition to a Gram stain.105,106 Negative fungal cultures may be found in 50% of cases and do not preclude a diagnosis of fungal disease.106,123 Fungal infections may be localized to one part of the vitreous and specimens obtained for culture may fail to sample these areas.123 Negative bacterial cultures may suggest the diagnosis of a fungal infection.
No consensus exists regarding treatment for patients with fungal endophthalmitis in the postoperative setting. Most reports of endophthalmitis postoperatively are retrospective reviews using various treatment options chosen on an individual patient basis.105,121,122 A combination of therapeutic options is usually employed including intraocular, intravenous, and oral antifungals, corticosteroids and PPV.91,105,106,122
Intraocular amphotericin B is usually the drug of choice for primary therapy until culture and sensitivity results are obtained.105,122 Amphotericin is effective against a wide range of fungal organisms capable of causing postoperative endophthalmitis.105,122 Pflugfelter and coworkers suggested that amphotericin B should be used after obtaining appropriate cultures if clinical evidence such as delayed onset of inflammation, inflammation in the presence of a relatively quiet eye, minimal discomfort, an inflammatory mass in the eye, or fungi on stained smear suggest a fungal infection.105 The drug should be administered in the area of maximum inflammation.105 Amphotericin may increase ocular inflammation after administration and may need to be given repeatedly if persistent or recurrent inflammation occurs.105,122,129 If intraocular amphotericin B therapy fails, miconazole treatment has been suggested.105 This may be true for Paecilomyces lilacinous, which has demonstrated resistance to amphotericin.105,106
Periocular antifungals have been suggested if the cornea, sclera, or anterior chamber is the source of infection.105 Animal studies have demonstrated good anterior chamber penetration of natamycin, amphotericin B, and miconazole following topical administration.96,130 Systemic antifungal use has been widely debated. No prospective controlled studies have evaluated the efficacy of systemic therapy for postoperative fungal endophthalmitis. Many patients receive systemic antifungals as part of their treatment regimen, but good visual outcomes have also been reported without systemic therapy.105,131 Systemic antifungal use is not without risk. Amphotericin B can induce renal toxicity and hematologic side effects and is often not tolerated. Ketoconazole and fluconazole may be better tolerated but still have the potential to induce liver toxicity.91,105,132 Flucytosine seems to be the least toxic, but it is generally not used for monotherapy because it is associated with a high incidence of resistance.105,133,135 The role of oral antifungal therapy in postoperative endophthalmitis has not been clearly defined, and the risks and benefits of their use must be weighed before treatment.
Corticosteroids must be used with caution in suspected cases of fungal endophthalmitis, and their role in treatment is still unclear. Steroids have been used in this setting in an effort to control inflammation and limit tissue destruction incited by the host inflammatory response.134 Pflugfelder and coworkers suggested that topical corticosteroids could be used to control inflammation after appropriate medical and surgical therapy had been employed.105 Shulman and Peyman reported a beneficial role for intraocular steroids in cases of fungal endophthalmitis.135
A surgical approach to postoperative fungal endophthalmitis is often undertaken.91,105,106,121,122 Vitrectomy including excision of involved ocular structures and intraocular injection of antifungals is suggested.105,106,122 Pflugfelder and coworkers reported that seven of eight patients with good visual results in their series had undergone vitrectomy followed by intraocular antifungal administration.105 This therapy may need to be repeated several times before the infection is controlled, and patients need to be monitored closely for recurrence despite removal of involved tissue.122
Bleb-Related Infection
Inflammation resulting from bleb-related infections is another cause of postoperative uveitis. Infection can develop in eyes after planned glaucoma filtering surgery or following inadvertent blebs after cataract surgery or trauma.136–139 Bleb-related infections can be further divided into localized bleb infection or bleb-associated endophthalmitis. The term blebitis was first coined by Brown and partners to describe a limited infection centered around a bleb without vitreous involvement.140 This is in contrast to bleb-related endophthalmitis, a more virulent infection with anterior chamber inflammation in addition to often-marked vitritis that typically develops months to years after glaucoma-filtering surgery, although cases of acute-onset, bleb-related endophthalmitis have been reported.137,141 Blebitis may be a precursor to the development of fulminant endophthalmitis.140,142 Treatment may be more effective when undertaken earlier; therefore, it is important to recognize bleb-related infections early on and to treat aggressively to avoid more serious ocular complications and a poorer visual prognosis.140
Clinical Features and Treatment
Blebitis and bleb-related endophthalmitis can be differentiated by the extent of ocular inflammation and differences in clinical presentation. Patients with blebitis typically present with a several-day history of redness, pain, photophobia, conjunctival discharge, and significant conjunctival injection surrounding an opalescent bleb (often with an overlying epithelial defect), which may contain inflammatory material.140–143 Patients may have variable anterior chamber reactions, hypopyon, and positive results to Seidel testing.140,141 By definition, patients with blebitis do not demonstrate vitritis. In contrast, patients with bleb-related endophthalmitis usually present with rapidly progressive symptoms including worsening visual acuity, pain, redness, diffuse conjunctival injection with an opalescent bleb (often without an overlying epithelial defect), anterior chamber reaction with variable fibrin or hypopyon and florid vitritis.137,141,142 Seidel testing may also be positive.137
Patients with blebitis develop infection in the setting of thin, cystic blebs, which typically leak on Seidel testing.140,142,143 Thin blebs lead to decreased tissue resistance and infection presumably develops when bacteria gain entry into the eye at the vulnerable bleb site and through overlying epithelial defects.142 This may explain why S. epidermidis and S. aureus, organisms that normally inhabit the ocular adnexa, are typically cultured from patients with blebitis.140–142 Blebitis often responds well to intense topical and subconjunctival antibiotic treatment and patients usually obtain nearly complete visual recovery, factors which may be due in part to the low virulence of the infecting organisms.137,140–142,144
Patients with bleb-associated endophthalmitis may develop infection in the setting of either thin or thick-walled blebs, with or without leakage.142 The organisms responsible for infection are usually more virulent bacteria, such as Streptococcus species or Haemophilus influenzae,101,137–139,141,142 which presumably gain entry to the anterior chamber by penetrating an intact conjunctival bleb.138,139,142,143,145 Endophthalmitis after inadvertent blebs is caused by similar organisms.141 Mandelbaum and colleagues reported a case of endophthalmitis occurring 60 years after inadvertent formation of a bleb.138 Rarely, bleb-associated endophthalmitis may develop in the acute postoperative period.137,139,141 In these instances, it is believed that perioperative introduction of bacteria, presumably from flora of the ocular adnexa, is responsible for infection.139,141,144 This would explain why coagulase-negative Staphylococci are the most common cause of acute bleb-related endophthalmitis, just as in cases of acute endophthalmitis following other ocular surgery.101,141
Treatment of bleb-related endophthalmitis is generally similar to that of acute postoperative endophthalmitis. Anterior chamber and vitreous cultures are usually obtained and intravitreal and periocular antibiotics administered. Vitreous specimens are more effective for identifying infectious organisms than cultures obtained from the aqueous or bleb; therefore, tapping the bleb is generally not recommended.137–139 Given the virulence of the infecting organisms, a vitrectomy is often required early in the treatment course and systemic antibiotics are often added, in contrast to postcataract endophthalmitis.138,139,141 Kangas and colleagues reported a final visual acuity of 20/400 or better in 55% of patients treated by initial PPV compared with 33% treated by initial vitreous tap without vitrectomy.137 Mandelbaum and associates have advocated that a complete vitrectomy be done as part of the initial therapeutic measures for eyes with bleb-associated endophthalmitis, given that a high proportion of these cases are caused by virulent bacteria that may severely damage the eye.138 Vitrectomy removes not only a significant bacterial inoculum but also damaging bacterial toxins and inflammatory factors induced by the infectious cascade.138,139
Risk Factors and Prognosis
In the last decade, the use of antifibrotic agents such as mitomycin C-(MMC) and 5-fluorouracil (5-FU) has increased.137 Histopathologic studies have shown that the use of MMC and 5-fluorouracil leads to thin-walled and cystic avascular blebs, which seem to be at greater risk for infection.146,147 These thin-walled blebs have a tendency to leak and studies have shown that bleb leakage is a significant risk factor for bleb-related infection.143,144,148 Wolner and colleagues found that the incidence of bleb-related endophthalmitis after trabeculectomy with 5-FU therapy resembled that of endophthalmitis after full-thickness glaucoma procedures, suggesting that the incidence of endophthalmitis after trabeculectomy with 5-FU is higher than that performed without adjunctive 5-FU.144 Bleb location may also be a risk factor. Higginbotham and partners found that inferior trabeculectomy performed with adjunctive MMC is associated with a greater than sevenfold increased risk of bleb-related endophthalmitis compared with superior filters with MMC.145 Wolner and colleagues noted a similar trend when 5-FU was used for inferior trabeculectomy. In their study, the relative risk of bleb-related endophthalmitis after inferior trabeculectomy with adjunctive 5-FU was 4.0 compared with a superior filter with 5-FU.144 Blebs associated with antifibrotic agents are thinner and may be more vulnerable to exposure and trauma induced by mechanical irritation from the lower lid margin.144 However, Mochizuki and partners found that the incidence of late bleb-related infection after trabeculectomy with antifibrotics was similar to that after trabeculectomy without these agents.143 Other risk factors that have been associated with an increased risk of bleb-related endophthalmitis include contact lens wear, conjunctivitis, and recent upper respiratory tract infections.138,139,149
Despite aggressive therapeutic maneuvers, patients with late bleb-related endophthalmitis typically have a poor visual prognosis.137–139,142,145,148 Kangas and associates reported a final visual acuity of 20/400 or better in 47% of patients following bleb-associated endophthalmitis.137 The poor visual outcome of patients is thought to result from the virulence of the infecting organisms.138,142,145 In the study by Kangas and associates, 40% of patients with streptococcal infection achieved a final acuity of 20/400 or better versus 52% in the nonstreptococcal group.137 Mandelbaum and colleagues reported that ten of sixteen eyes with Streptococcus-related endophthalmitis lost all light perception and fared worse than those without such infection.138 These results underscore the importance of early and aggressive treatment in patients with suspected bleb-related inflammation.