The mechanisms of ocular and adnexal involvement include active infection and immunogenic disease.
Active infection can occur as a primary ocular infection caused by introduction into the eye by contaminated hands or fomites, or exposure to dust or sputum particles laden with bacilli.37 More commonly, active ocular or adnexal infections occur secondarily by hematogenous spread of tubercle bacilli from a distant site of active primary or secondary stage systemic disease or by contiguous spread (e.g., nasal sinuses, meninges). The result is granuloma (tubercle) formation that can involve any part of the eye, orbit, or visual pathways.
EYELIDS
Primary infection of the eyelid is characterized by ulceration and lymphadenopathy. However, lid involvement usually occurs as lupus vulgaris, a slowly progressive, chronic process without regional lymphadenopathy, which begins as a small tubercle under the epithelium. The nodule becomes more superficial in location, varying in size from pinpoint to pea-sized, with surrounding erythema.38 The nodules increase in numbers, forming a lupus-patch. Large areas can become involved and result in severe cicatrization, causing ectropion and corneal exposure.
ORBIT
Orbital involvement can include cellulitis, periostitis, osteomyelitis abscess formation, and chronic dacryoadenitis. Tuberculous orbital involvement may displace the globe, cause irregular destruction of the orbital walls, and induce hyperostosis.39 These lesions usually are of metastatic origin. Fistula formation can occur.40 Dacryocystitis can be primary or secondary to adjacent osseous TB.
CONJUNCTIVA
Tuberculosis of the conjunctiva is very rare. Infection can be primary, in which the source is exogenous (e.g., airborne particles, trauma). Secondary infection can be endogenous via the bloodstream or by direct extension from an adjacent focus. Bulbar conjunctival nodules in a patient with a history of TB should make one suspicious of conjunctival TB.41 Conjunctivitis may start slowly as a granulomatous lesion or present as an acute purulent or pseudomembranous conjunctivitis. Primary conjunctivitis can be accompanied by visible lymph node involvement, that is, Parinaud's oculoglandular syndrome. Gross lymph node involvement is not typical of secondary infections42 but may occur.43
CORNEA
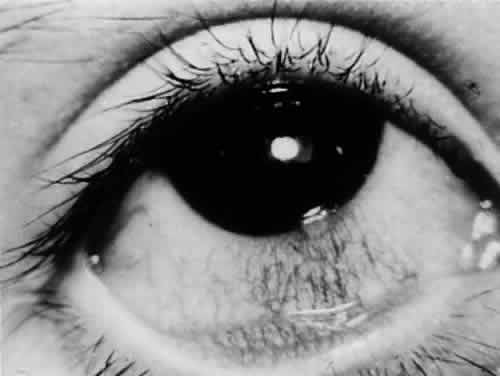
Corneal involvement usually is allergic in origin (phlyctenulosis, interstitial keratitis) or is secondary to spread from adjacent structures (sclerokeratitis). Primary infectious keratitis has been described but is rare. Phlyctenular keratoconjunctivitis is the most common form of external ocular TB (Fig. 1). Phlyctenulosis may be caused by tubercle protein hypersensitivity.44
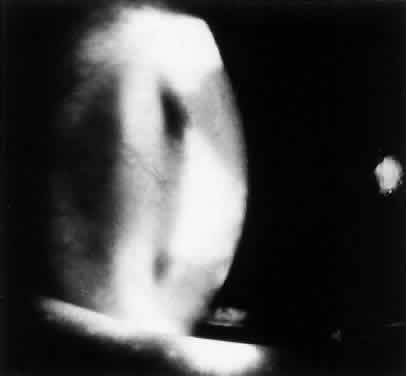
Interstitial keratitis typically affects one eye (Fig. 2). The tuberculoproteins of the cell wall of M. tuberculosis are responsible for causing allergic reactions in the cornea, producing interstitial keratitis.45 The clinical course is prolonged and may involve frequent attacks of inflammation. Infiltration in TB usually is peripheral and sectoral and spares the central cornea (Fig. 3). Often there is associated scleritis. TB usually affects the superficial and middle layers of corneal stroma; vascularization follows, with the vessels usually located in the anterior stroma. Residual localized opacification ensues because of necrosis in areas of infiltration. These features are in contrast to luetic interstitial keratitis, which involves the stroma more posteriorly, affects the central and peripheral cornea, and tends to leave less opacification because corneal infiltration usually is not as severe.46
|
|
Primary corneal keratitis caused by M. tuberculosis varies in clinical appearance, and thus, diagnosis is difficult to make on clinical grounds.47 Secondary keratitis can result from direct spread from tuberculous scleritis or anterior segment infection.48
SCLERA AND EPISCLERA
Episcleritis is the result of hypersensitivity reaction to tuberculoprotein. Duke-Elder49 described epibulbar disease to be either ulcerative, nodular, hypertrophic papillary, or polypoid. Deep nodular scleritis results from direct invasion of the sclera by the tubercle bacillus. Tuberculous scleritis usually elicits pain as a prominent feature, as is seen typically in scleritis associated with autoimmune diseases. Clinically, the scleritis consists of one or more indurated nodules that appear fixed to the sclera and are associated with marked injection. Eventually, the nodule turns yellow as it undergoes caseation, and eventually ulceration follows. Involvement of the adjacent ciliary body and iris can result in an associated granulomatous anterior uveitis. Scleral perforation may occur.50,51
The scleritis is refractory to treatment with topical corticosteroids, suggesting that the scleritis is the result of direct invasion of the tubercle bacillus and is not a hypersensitivity reaction.52
UVEITIS
Tuberculosis once was considered a common cause of uveitis, but it currently is uncommon. Its incidence accounts for about 0.2% of all uveitis cases. Anterior uveitis usually is granulomatous and is characterized by mutton-fat keratic precipitates, Koeppe and Busacca nodules on the iris. Hypopyon can occur. Less commonly, the anterior uveitis can be nongranulomatous, with an acute, recurrent, or chronic course. Topical corticosteroids may convert an anterior granulomatous uveitis to a nongranulomatous form.53 Conglomerate tubercles of the iris resulting from coalescing of miliary tubercles of the iris rarely are seen but have been reported.54 The possibility of TB should be entertained for any steroid nonresponsive uveitis.
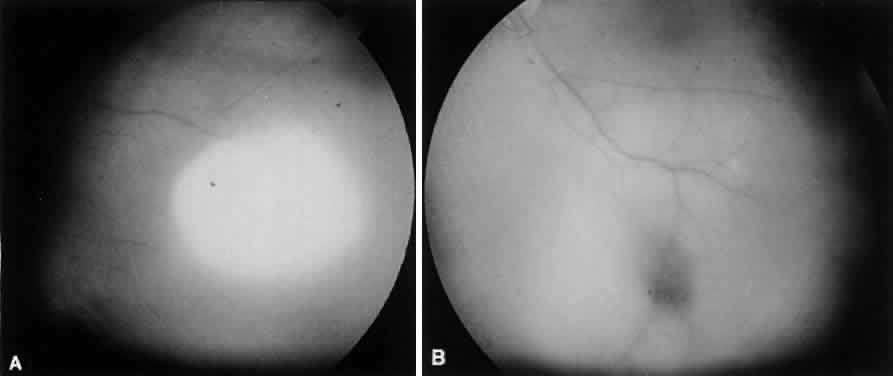
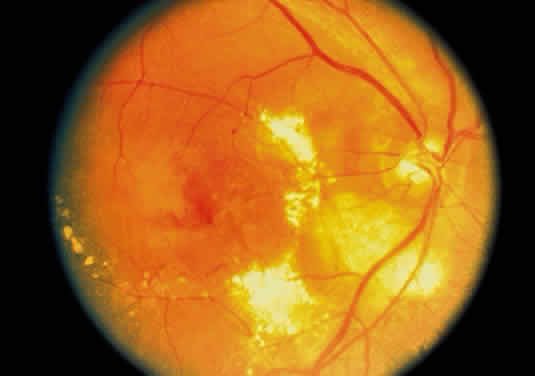
The choroid has a particular propensity for involvement by TB. Choroidal granulomas can be solitary or—as in miliary TB—multiple, causing a disseminated choroiditis. Choroidal granulomas are yellowish-white nodules with indistinct borders, ranging in diameter size from 0.5 mm to 2 cm. They may be seen in patients without active systemic disease (Figs. 4 and 5). Although A-scan findings are variable, B-scan typically reveals a solid elevated choroidal mass with an absent scleral echo due to absorption by inflammatory cells.55 Fluorescein angiography generally shows early hyperfluorescence with leakage at the margins and late central leakage. After treatment, there may be loss of early hyperfluorescence and late staining. Choroidal neovascularization can occur.55–57 Endobiopsy has been performed safely for diagnosis.58
|
Exudative retinitis and retinal vasculitis can follow. Involvement of the posterior uvea sometimes can resolve spontaneously. The resulting chorioretinal scars could be confused with old toxoplasmosis or histoplasmosis scars and may lead to loss of central vision.59 Serum angiotensin-converting enzyme often is measured to investigate possible sarcoidosis but may be positive in 38.9% of miliary TB cases.60 Care should be taken because these diseases may be confused clinically.
Tuberculous choroiditis is more common in patients with AIDS than in the general population.61,62 Vitritis may be absent.13 Other causes of choroidal nodules in patients with AIDS are Pneumocystis carinii, fungi, atypical mycobacteria, syphilis, and lymphoma. Uveitis also may be caused by the use of rifabutin for MAI.63
Almenoff et al64 demonstrated mycobacterial cell wall-deficient forms in 19 of 20 patients with sarcoid and none of 20 controls. This and other similar work suggests that mycobacteria may play a broader role in etiologies of uveitis than previously suspected.
RETINA
Tuberculosis of the retina may occur from endogenous spread from a distant source. The inflammation commences in the vessel layer of the retina and may take two forms. In the miliary type, there is formation of small tubercles that remain localized and eventually heal (superficial exudative retinitis). In the second form, there may be massive retinitis with extensive grey-white lesions and heavy vitreous reaction. Severe endophthalmitis can ensue. Care should be taken to distinguish these lesions from retinoblastoma in children.54,65,66 Retinal periphlebitis may result from direct infection of the retina by tubercle bacilli.44 This has been reported to cause a central retinal vein occlusion.67 Disc swelling, macular star, and retinal folds may occur in a neuroretinitis pattern.25 TB often affects the retina by spread from adjacent involved choroid. The choroid usually is involved in miliary TB.68 Eale's disease may result from hypersensitivity to tuberculin protein and other antigens.44
OPTIC NERVE
Optic nerve involvement may accompany uveitis or tuberculous meningitis, either as a direct infiltration of the nerve, as an inflammatory disc edema, or bilaterally because of increased intracranial pressure (papilledema). Optic atrophy after TB meningitis may be more common in patients with cerebrospinal fluid protein content greater than 75 mg/dL.69 The addition of dexamethasone to the treatment regimen of tuberculous meningitis may help prevent optic atrophy. Optic neuropathy also may occur as a result of antituberculous treatment with ethambutol and INH.
CENTRAL NEUROOPHTHALMIC PATHWAYS
Tuberculous meningitis typically causes a basilar meningitis and may involve any of the cranial nerves. It should enter into the differential diagnosis whenever there is a pattern of multiple cranial nerve involvement. Alternatively, disease within the CNS may lead to focal cranial mononeuropathies or other neuroophthalmic syndromes, such as internuclear ophthalmoplegia,71 bilateral internuclear ophthalmoplegia,72 chiasmal or junctional visual loss,73,74 gaze palsy,75 and third cranial nerve palsy.69
A tuberculoma may behave as a space-occupying lesion and has been reported involving the cavernous sinus.76 CNS TB must make one vigilant for local ocular disease.77 Conversely, ocular disease must make one aware of the possibility of CNS involvement. The ophthalmologist may be of great assistance in diagnosing systemic or miliary TB in the choroid in critically ill patients when laboratory support is unavailable or delayed.59