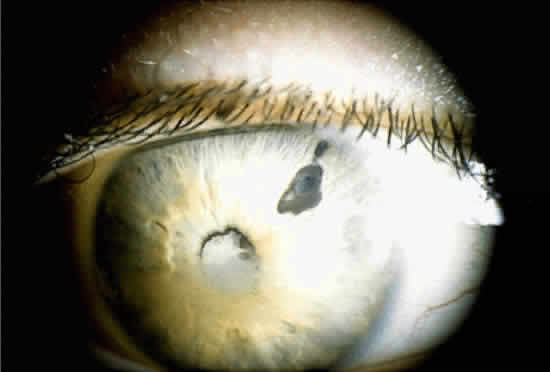
When a cataract develops in a uveitis patient, the management is more complex than in the nonuveitis patient. The presence of posterior synechiae, pupillary membranes, and inflammation may make the surgery more difficult, and the postoperative course is often stormy (Fig. 1). Much controversy still exists regarding the best method of managing the uveitis patient with a cataract. Ideally, absolute control of inflammation should be obtained for at least 3 months preceding surgery; however, there are exceptions to this rule, as in the case of lensinduced uveitis.9
The choice of surgical technique is also controversial, but it is probably not as important as the perioperative management. The patient should be treated preoperatively with topical steroid drops (e.g., prednisolone acetate or phosphate) at least four times a day for at least 3 days before surgery. Also, we often pretreat with an oral steroid (usually prednisone 1 mg/kg/day) for 3 days, if the patient's medical status permits.
Adequate pupillary dilation is often difficult to achieve preoperatively because of posterior synechiae and pupillary membranes. If this is the case, a laser peripheral iridotomy can be performed preoperatively, or a surgical peripheral iridectomy can be performed at the time of surgery. The posterior synechiae can then be lysed with the aid of a cyclodialysis spatula; access is gained to the superior ones through the peripheral iridotomy. If adequate pupillary dilation is still not achieved, straight or curved long-handled retinal scissors can be used to fashion small membranotomies or sphincterotomies (1 to 2 per clock hour), and viscoelastic material can then be used to dilate the pupil. This technique has an advantage over a single large sphincterotomy in that the pupil usually remains functional afterward. If adequate pupillary dilation is still not achieved, iris hooks can be used to further dilate the pupil. In some cases, iris hooks provide sufficient dilation without the need for sphincterotomies. The lens is then removed with the use of either phacoemulsification or standard extracapsular techniques.
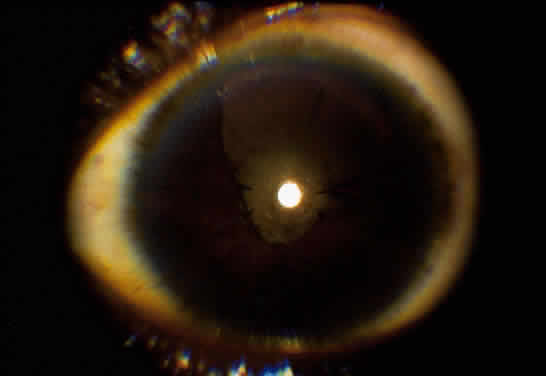
Implantation of an intraocular lens (IOL) is still controversial. There is much evidence that an IOL can be safely implanted in cases of Fuchs' iridocyclitis10–12 and pars planitis.8,13,14 In contrast, most evidence in cases of children with JRA is in favor of lensectomy/vitrectomy with no IOL (Fig. 2).4,15–17 The use of an IOL in uveitis of other etiologies is still uncertain, but an IOL should never be inserted in a patient with inflammation that could not be adequately controlled preoperatively. If an IOL is to be inserted, we prefer an all polymethyl-methacrylate lens to one with polypropylene haptics, because this may, at least in theory, lessen the chance of postoperative inflammation.12,17 Recently, in selected cases, we had good postoperative results using foldable acrylic lenses. Placement of the IOL in the capsular bag rather than the ciliary sulcus is also preferred because this may lessen the risk of inflammation secondary to iris-haptic contact.
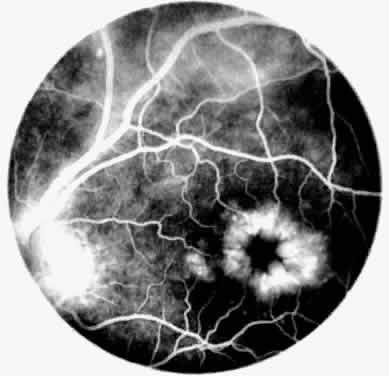
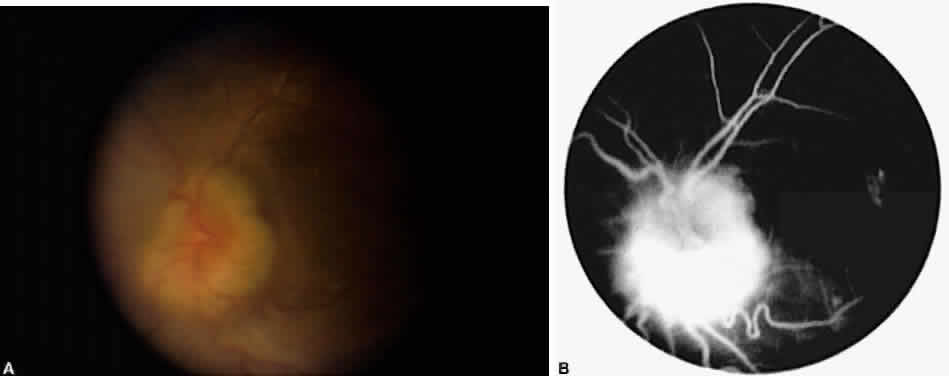
Occasionally, even in eyes with little or no preoperative inflammation, significant membranes form around an IOL (Fig. 3). This can occur even in the absence of significant anterior chamber cell and flare in the postoperative period.18 Although these cocoonlike membranes can be lysed with the Nd:YAG laser, they tend to reform. Therefore, it is imperative to be vigilant in the first 6 to 12 weeks after surgery, aggressively treating any signs of inflammatory deposits on the IOL, even in the absence of anterior chamber reaction. If laser membranectomy is required, vigorous anti-inflammatory treatment is necessary to prevent re-formation of the membrane. In severe cases, removal of the IOL may be required.
It has been suggested that pars plana vitrectomy may be warranted when substantial vitreous cell and debris might preclude good postoperative visual acuity.4,8,12,19 Although we do not routinely perform pars plana vitrectomy on all uveitis patients with cataract, this procedure is considered for eyes with significant vitreous debris or inflammation.
The postoperative management is similar to that of nonuveitis patients, except that inflammation is usually more severe and prolonged, often requiring depot steroid injections and systemic anti-inflammatory treatment.
The results of cataract surgery in uveitis patients vary according to the preoperative diagnosis. Patients with Fuchs' iridocyclitis generally do well, with a visual acuity of 20/40 or better being the rule.10–12 Patients with pars planitis also do well, with 60% to 82% achieving a visual acuity greater than 20/40.8,18 Most pars planitis patients who fail to achieve good visual acuity do so because of cystoid macular edema.4,8,9 In these patients, perhaps more aggressive anti-inflammatory treatment, both preoperatively and postoperatively, will improve visual outcome.
Patients with JRA do not tend to have as good an outcome as those with Fuchs' or pars planitis; a visual acuity greater than 20/40 is generally achieved in only about 60% of patients.15 However, one study using aggressive preoperative control of inflammation has reported a visual acuity greater than 20/40 in 75% of JRA patients.20 Finally, those patients with idiopathic and other forms of nongranulomatous anterior uveitis tend to do well postoperatively, with almost 80% achieving a visual acuity of 20/40 or better, again provided inflammation is well controlled preoperatively.9