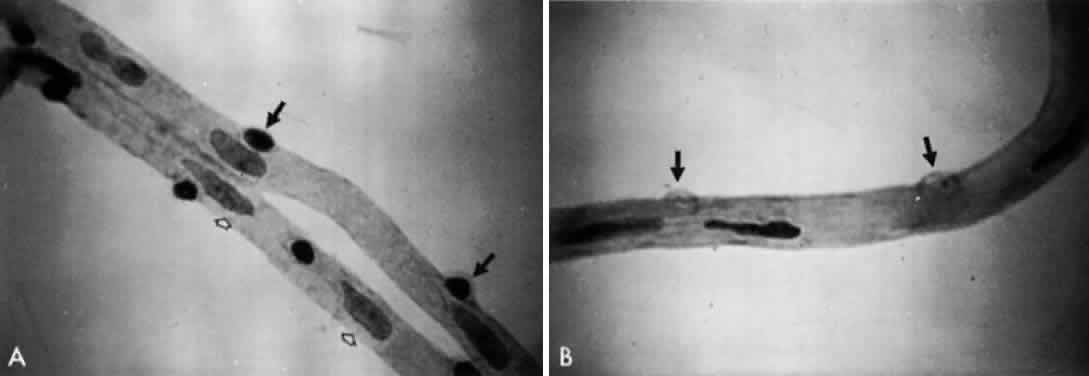
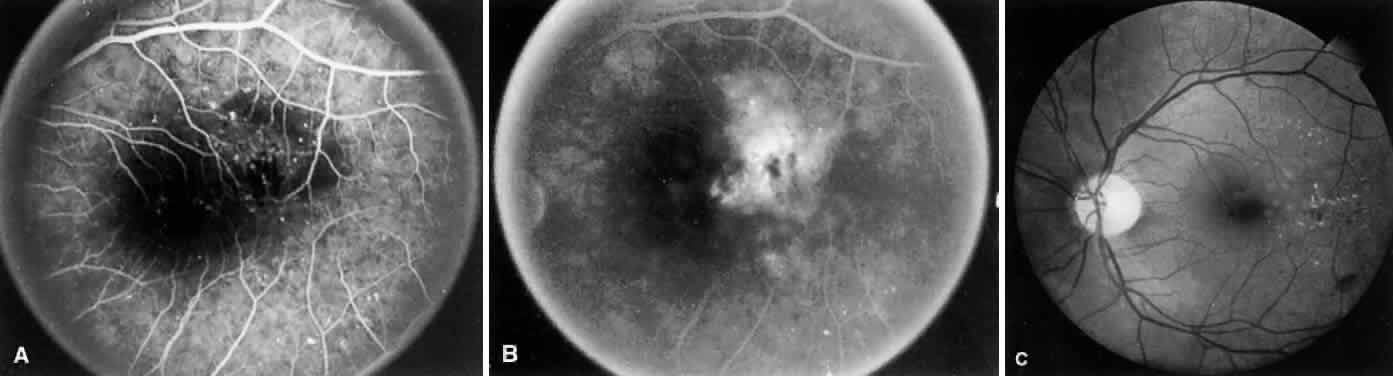
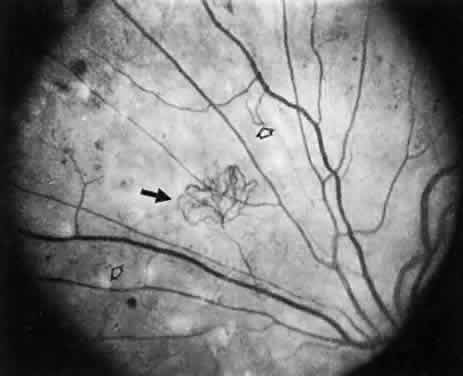
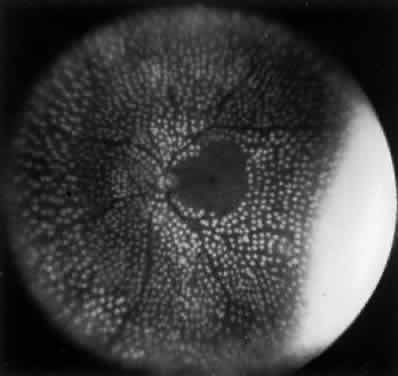
1. Operational Research Department, National Society to Prevent Blindness: Vision
Problems in the U.S.: A Statistical Analysis. New York, National
Society to Prevent Blindness, 1980 2. National Diabetes Data Group: Diabetes in America, NIH publication no. 95-1468. Washington, DC, National Institutes of Health, 1995 3. World Health Organization: Diabetes Mellitus: Report of a WHO Study Group, technical
report #727. Geneva, World Health Organization, 1985 4. King H, Rewers M: Global estimates for prevalence of diabetes mellitus and impaired glucose
tolerance in adults. Diabetes Care 16:157, 1993 5. Alberti KG, Mazza ER (eds): Frontiers of Diabetes Research: Current Trends
in NIDDM. Amsterdam, Elsevier, 1989 6. Gruber W, Lander T, Leese B (eds): Diabetes Health Economics Study Group: The
Economics of Diabetes and Diabetes Care. Geneva, International
Diabetes Federation and World Health Organization, 1997 7. National Society to Prevent Blindness: Vision Problems in the U.S. Data
Analysis: Definitions, Data Sources, Detailed Data Tables, Analysis, Interpretations. New
York, National Society to Prevent Blindness, 1980 8. Cruickshanks KG, Zadheim CM, Moss SE: Genetic marker associations with proliferative retinopathy in persons diagnosed
with diabetes before thirty years of age. Diabetes 41:879, 1992 9. Klein R, Klein BEK, Moss S: The Wisconsin Epidemiology Study of Diabetic Retinopathy. II. Prevalence
and risk of diabetic retinopathy when age at diagnosis is less than 30 years. Archiv Ophthalmol 102:520, 1984 10. Klein R, Klein BEK, Moss S: The Wisconsin Epidemiology Study of Diabetic Retinopathy. III. Prevalence
and risk of diabetic retinopathy when age at diagnosis is 30 or more
years. Archiv Ophthalmol 102:527, 1984 11. Klein R, Klein BEK, Moss SE: Is blood pressure a predictor of the incidence of progression of diabetic
retinopathy? Archiv Intern Med 149:2427, 1989 12. The Diabetes Control and Complications Trial Research Group: The effect
of intensive treatment of diabetes on the development and progression
of long term complications in insulin dependent diabetes mellitus. N
Engl J Med 329: 977, 1993 13. United Kingdom Prospective Diabetes Study [UKPDS] Group: Intensive
blood-glucose control with sulphonylureas or insulin compared with
conventional treatment and risk of complications in patients with type
II diabetes. Lancet 352:837, 1998 14. Brownlee M, Cerami A, Vlassara H: Advanced glycation end products in tissue and the biochemical basis of
diabetic complications. N Engl J Med 318:1315, 1988 15. Vlassara H, Brownlee M, Manogue K: Advanced glycation end product (AGE) binding to its macrophage receptor
stimulates of a multifunctional growth promoting monokine. Science 240:1546, 1988 16. Cogan DG, Toussaint D, Kuwabara T: Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol 66:366, 1961 17. Cogan DG, Kinoshita JH, Kador PF et al: Aldose reductase and complications of diabetes. Ann Intern Med 101:82, 1984 18. Sorbinil Retinopathy Trial Research Group: A randomized trial of sorbinil, an
aldose reductase inhibitor, in diabetic retinopathy. Arch Ophthalmol 108:1234, 1990 19. Feman SS, Leonard-Martin TC, Redman: The Vanderbilt Classification System in the evaluation of diabetic retinopathy
patients treated with Alredase. Trans Am Ophthalmol Soc 94:433, 1996 20. The Diabetes Control and Complications Trial Research Group: The rleationship
of glycemic exposure (HbA1c) to the risk of development and progression
of retinopathy in the diabetes control and complications trial. Diabetes 44:968, 1995 21. Aiello LP, Northrup JM, Keyt BA: Hypoxic regulation of vascular endothelial growth factor in retinal cells. Arch Ophthalmol 113:1538, 1995 22. Schwartz DE: Corneal sensitivity in diabetics. Archiv Ophthalmol 91:174, 1974 23. Friend J, Thoft RA: The diabetic cornea. Int Ophthalmol Clin 24:111, 1984 24. Schultz RO, Peters MA, Sobocinski K: Diabetic corneal neuropathy. Trans Am Ophthamol Soc 81:107, 1983 25. Tsubota K, Chiba K, Shimazaki J: Corneal epithelium in diabetic patients. Cornea 10:156, 1991 26. Taylor HR, Kimsey RA: Corneal epithelial basement membrane changes and type. Invest Ophthalmol Vis Sci 20: 548, 1981 27. Parrish CM: The cornea in diabetes mellitus. Feman SS (ed): Ocular Problems
and Diabetes Mellitus, p 179. Boston, Blackwell Scientific, 1992 28. Goebbels M, Spitznas M, Oldendoerp J: Impairment of corneal epithelial barrier function in diabetics. Graefes Arch Clin Exp Ophthalmol 227:142, 1989 29. Busted N, Olsen T, Schmitz O: Clinical observations on the corneal thickness and the corneal endothelium
in diabetes mellitus. Br J Ophthalmol 65:687, 1981 30. Schultz RO, Matsuda M, Yee RW, Edelhauser HS: Corneal endothelium changes in type I and type II diabetes mellitus. Am J Ophthalmol 98:401, 1984 31. Shetlar DJ, Bourne DM, Campbell J: Morphologic evaluation of Deseemet's membrane and corneal endothelium
in diabetes mellitus. Ophthalmology 96:247, 1989 32. Waring GO III: Examination and selection of patients for refractive keratotomy. In
Waring GO III (ed): Refractive Keratotomy for Myopia and Astigmatism, p 309. St
Louis, Mosby Year Book, 1992 33. Pallikaris IG, Siganos DS: Excimer laser in situ keratomileusis and photorefractive keratectomy for
correction of high myopia. J Refract Corneal Surg 10:498, 1994 34. Becker B: Diabetes mellitus and primary open-angled glaucoma. Am J Ophthamol 71:1, 1971 35. Klein BE, Klein R, Jensen SC: Open-angle glaucoma in older onset diabetes: The Beaver Dam Eye Study. Ophthalmology 101:1173, 1994 36. Michaelson IC: The mode of development of the vascular system of the retina with some
observations on its significance for certain retinal diseases. Trans Ophthalmol Soc UK 68:137, 1949 37. Aiello LP, Avery RL, Arrig PG: Vascular endothelium growth factor in ocular fluid of patients with diabetic
retinopathy and other retinal disorders. N Engl J Med 331:1480, 1994 38. Shields MB: Glaucoma in diabetic patients. Feman SS (ed): Ocular Problems
and Diabetes Mellitus, p 207. Boston, Blackwell Scientific, 1992 39. Molteno ACB, VanRooyen MNB, Bartholomew RS: Implants for draining neovascular glaucoma. Br J Ophthalmol 61:120, 1977 40. Ederer F, Hiller R, Taylor HR: Senile lens change in diabetes in two population studies. Am J Ophthalmol 91:381, 1981 41. Flanagan DW: Current management of established diabetic eye disease. Eye 7:302, 1993 42. Feman SS: The natural history of the first clinically visible features of diabetic
retinopathy. Trans Am Ophthalmol Soc 92:744, 1994 43. Early Treatment Diabetic Retinopathy Study Group: Photocoagulation for
diabetic macula edema. Arch Ophthalmol 103:1796, 1985 44. Diabetic Retinopathy Study Research Group: Preliminary report on effect
of photocoagulation therapy. Am J Ophthalmol 81:383, 1976 45. Diabetic Retinopathy Study Research Group: Four risk factors for severe
visual loss in diabetic retinopathy. Arch Ophthalmol 97:654, 1979 46. Ferris F: Early photocoagulation in patients with either type I or type II diabetes. Trans Am Ophth Soc 94:505, 1996 47. Kalebic J, Garbisa S, Glaser B, Liotta L: Basement membrane degradation by migrating endothelial cells. Science 221:281, 1983 48. Davis M: Vitreous contraction in proliferative diabetic retinopathy. Arch Ophthalmol 54:741, 1965 49. Wallow IHL, Geldener DS: Endothelial fenestrae in proliferative diabetic retinopathy. Invest Ophthalmol Vis Sci 19:1176, 1980 50. Tagawa H, McMeel JW, Furukawa H: Role of the vitreous in diabetic retinopathy. Ophthalmology 93:596, 1986 51. Diabetic Retinopathy Vitrectomy Study Research Group: Early vitrectomy
for severe proliferative diabetic retinopathy in eyes with useful vision. Ophthalmology 95:1307, 1988 52. Diabetic Retinopathy Vitrectomy Study Research Group: Early vitrectomy
for severe vitreous hemorrhage in diabetic retinopathy: Four year results
of the randomized trial. Arch Ophthalmol 108:958, 1990 53. Diabetic Retinopathy Vitrectomy Study Research Group: Early vitrectomy
for severe proliferative diabetic retinopathy in eyes with useful vision: Clinical
application of results of the randomized trial. Ophthalmology 95:1321, 1988 54. Feman SS, Leonard-Martin TC, Semchyshyn TM: The topographic distribution of the first sites of diabetic retinal neovascularization. Am J Ophthalmol 125:704, 1998 55. Moss SE, Klein R, Kessler SD, Richie KA: Comparison between ophthalmoscopy and fundus photography in determining
the severity of diabetic retinopathy. Ophthalmology 92:62, 1985 56. American Diabetes Association: Eye care guidelines for patients with diabetes
mellitus. Diabetes Care 11:745, 1988 57. American Academy of Ophthalmology, Quality of Care Committee, Retinal Panel: Diabetic
Retinopathy: Preferred Practice Pattern. San Francisco, American
Academy of Ophthalmology, 1989 58. Rubin RJ, Altman WM, Mendelson DN: Health care expenditures for people with diabetes mellitus. J Clin Endocrinol Metab 78:809, 1994 59. Javitt JC, Canner JK, Sommer A: Cost effectiveness of current approaches to the control of retinopathy
in type I diabetes. Ophthalmology 96:255, 1989 60. Javitt JC, Aiello LP, Bassi LJ: Detecting and treating retinopathy in patients with type I diabetes mellitus: Savings
associated with improved implementation of current guidelines. Ophthalmology 98:1565, 1991 61. Javitt JC, Aiello LP, Chang YP: Preventive eye care in people with diabetes is cost saving to the federal
government: Implications for health care reform. Diabetes Care 17:909, 1994 62. American Diabetes Association: Policy statement on diabetic retinopathy: Clinical
practice recommendations. Diabetes Care 21(suppl 1):157, 1998 |