INTRACRANIAL DISORDERS
Pituitary adenomas and meningiomas do appear to become more symptomatic and grow more rapidly during pregnancy, although intracranial tumors overall do not appear to be more frequent than in nonpregnant women.60,61 Because some of the symptoms of intracranial processes, such as nausea and vomiting, may be related to pregnancy alone, there may be a delay in diagnosing intracranial disorders. Intracranial disorders should be included in the differential diagnosis of pregnant women with visual acuity or visual field loss, persistent headaches, or oculomotor palsies,62,63 while realizing that other processes may be responsible as well.
Pituitary Adenoma
Asymptomatic pituitary adenomas are common. A recent meta-analysis estimated the prevalence of pituitary adenomas at 16.7% (14.4% in autopsy studies and 22.5% in radiologic studies).69 Historically, these tumors have been characterized based on size with microadenomas measuring less that 1 cm and macroadenomas measuring greater than or equal to 1 cm. Although pituitary adenomas are now further classified according to immunohistochemistry and functional status, the risk of visual changes in pregnant women with pituitary adenomas is related primarily to tumor size.
The availability of drugs such as bromocriptine and cabergoline to induce ovulation in women with amenorrhea and amenorrhea allow many women with pituitary adenomas to conceive.88 Pituitary adenomas can enlarge during pregnancy, probably as a result of a combination of the physiologic enlargement seen in normal pregnant women and growth of tumor. An MRI study of 17 pregnancies in women with prolactinomas reported no change in size in 45%, increased size in 27%, and decreased size in 27%.72 Another investigation estimated the risk of adenoma growth during pregnancy at 1% for microadenomas and 23% for macroadenomas.73 The critical clinical issue for the ophthalmologist is whether this enlargement is associated with visual symptoms and signs, which then warrant treatment during pregnancy.
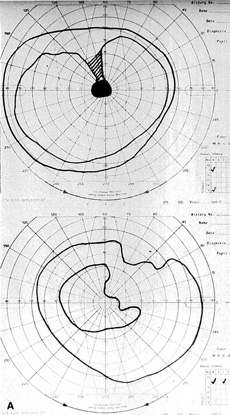
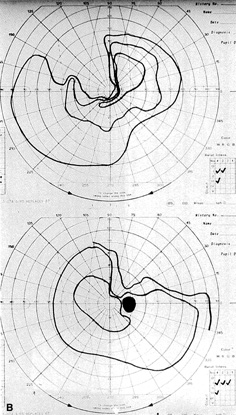
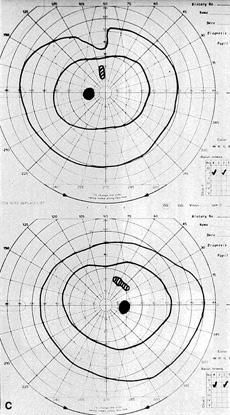
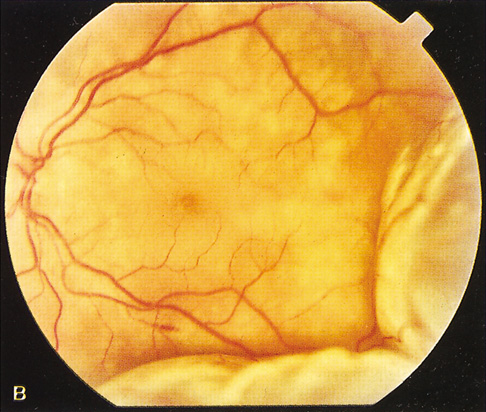
The most common visual findings are bitemporal visual field defects and visual acuity decrease (Fig. 1),78 but other visual disturbance, headaches, and rare diplopia also may be seen.74,75 The risk of a woman developing visual loss during pregnancy primarily is related to tumor size. A cohort study of 111 pregnancies in 65 women found that six of eight (75%) with macroadenomas, whereas none of 57 (0%) with microadenomas developed vision loss during pregnancy.70 A smaller study of four women with macroprolactinomas similarly showed irreversible visual field loss in three of four (75%).71 A third study found a rate of visual involvement in five of 91 (5.5%) with microadenomas and 36% of macroadenomas.76 In contrast, one study reported that only one of 14 pregnant women with large prolactinomas developed visual field defects during pregnancy.77 A final study reported that four of 18 (22%) with adenomas developed visual loss but tumors were not differentiated according to size.78
There is often spontaneous regression of the adenoma postpartum (see Fig. 1), so that the decision to treat depends on the severity of visual findings during pregnancy, and how early in pregnancy the findings developed.74 Fortunately, the dopamine agonists bromocriptine and cabergoline can cause tumor regression both within and outside of the context of pregnancy. Many studies have demonstrated the efficacy of bromocriptine for pregnancy-associated adenoma progression79–85 and there have not been any reports of adverse pregnancy outcomes related to the drug.83–85 Cabergoline also appears to be effective86,87 and safe87 during pregnancy. One case report describes the successful treatment of a thyrotropin secreting macroadenoma during pregnancy with octreotide resulting in improved visual fields, reduced tumor size, and good pregnancy outcome. Surgical therapy of symptomatic adenomas also has been successfully employed during pregnancy.80
Because the risk of symptomatic progression of a microadenoma during pregnancy is small, discontinuation of dopamine agonist therapy should be considered once pregnancy is confirmed. Treatment options for patients with macroadenomas include discontinuation of dopamine agonist therapy when pregnancy is confirmed and reinstituting therapy if the patient develops vision loss, continuous dopamine agonist therapy throughout pregnancy, and surgical debulking of the tumor prior to pregnancy.89 Decisions regarding management should be guided by an ophthalmologist, endocrinologist, obstetrician, radiologist, and possibly a neurosurgeon.
Intracranial Meningioma
Sixty-five percent of all intracranial meningiomas occur in women.192 Growth of these tumors is typically slow, producing insidious visual disturbances. In contrast, during pregnancy some meningiomas rapidly enlarge producing dramatic and relatively acute vision loss193,194 The diagnosis can be delayed because nausea, vomiting, and other symptoms caused by the tumor may be attributed to pregnancy.193 The tumors present more commonly during the second half of pregnancy.61 The predominance of meningiomas in women, their accelerated growth during pregnancy and the luteal phase of menstruation, and an association with breast cancer led to studies examining the potential role of hormones on meningioma growth.200 Subsequent studies have supported this association by demonstrating that about 70% of meningiomas express progesterone receptors and approximately 30% express estrogen receptors.201
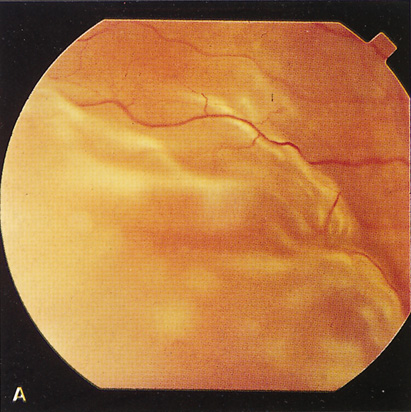
Ocular findings can include decreased visual acuity, visual field defects that depend on the location of the tumor, oculomotor palsies, papilledema, and late optic atrophy.195–199 Symptoms may abate spontaneously after delivery and can recur during subsequent pregnancies.193,202 Hormone-responsive intracranial meningiomas should be considered in the differential diagnosis of visual disturbances during pregnancy.193 Close cooperation among ophthalmologist, neurosurgeon, obstetrician, and neonatologist are essential for an optimal outcome.
The treatment of choice during pregnancy is usually surgical excision,193 but brachytherapy has been decribed203 and external beam radiation may be considered. Antiprogesterone therapy may be effective in receptor positive cases but is unlikely to have a role during pregnancy.
Lymphocytic Hypophysitis
Lymphocytic hypophysitis (LH) or adenohypophysitis is a rare autoimmune condition that occurs most frequently in women in the last trimester of pregnancy or the early postpartum period. The most common presenting symptoms are visual field defects and headache. Most patients show signs of isolated or multiple pituitary hormone deficiencies. Adrenocorticotropin hormone (ACTH) secretion is most frequently impaired, followed by thyroid stimulating hormone (TSH) gonadotropins, growth hormone (GH), and prolactin (PRL). One-third of the cases involve hyperprolactinemia.90
One study of 16 patients found that 63% had anterior pituitary hypofunction, 56% had symptoms consistent with an expanding pituitary mass, 38% had hyperprolactinemia, 25% had associated autoimmune thyroiditis, and 19% had diabetes insipidus. Computed tomography (CT) and MRI scanning demonstrated a pituitary mass mimicking an adenoma in 83% of cases.91
Because LH can present with headache, visual field defects, hyperprolactinemia, and a pituitary mass by imaging, this disorder can be difficult to distinguish clinically from a prolactinoma. However, this differentiation is critical because a failure to recognize LH can lead to untreated pituitary insufficiency and death92 and an incorrect diagnosis of pituitary adenoma may lead to unnecessary transsphenoidal surgery. A study of 45 patients with lymphocytic hypophysitis and 806 with pituitary adenoma during pregnancy found that history and endocrinologic evaluation will usually differentiate these disorders. The spontaneous pregnancy rate was 100% in LH, whereas only 2.4% in patients with pituitary adenoma. Prolactin levels averaged 34.6 in LH compared with 393 for pituitary adenomas. Hypothyroidism and hypocortisolism were present in 57.5% of LH cases but only 2.5% of patients with pituitary adenomas. There were no distinguishing features on radiographic studies.93
The authors recommend that patients presenting to an ophthalmologist during pregnancy with signs and symptoms consistent with a pituitary disorder be co-managed with an endocrinologist, obstetrician, radiologist, and possibly a neurosurgeon.
There is strong anecdotal evidence in the form of case reports that treatment with steroids can lead to marked improvement in vision loss from LH and possibly avoid permanent pituitary insufficiency in some cases.94–98 Most cases lead to varying degrees of irreversible visual field loss or pituitary insufficiency. However, there are five reported cases of complete recovery98–102 and only one of those cases was treated with steroids.98
Little was known about the etiology of LH until recently. Serum autoantibodies directed against a 49-kDa cytosolic protein had been detected by immunoblotting in 70% of patients with biopsy proven LH. In 2002, the target pituitary autoantigen was identified as alpha-enolase103 and similar autoantigen targeting of the gamma-enolase isoform found in placental tissue was soon found.104 This established a direct link between pituitary and placental autoantigens and provides a theoretical basis for the strong predilection for LH to occur during or shortly after pregnancy. The authors are not aware of any evidence suggesting increased perinatal morbidity or mortality associated with LH.
Idiopathic Intracranial Hypertension (Benign Intracranial Hypertension or Pseudotumor Cerebri)
Idiopathic intracranial hypertension (IIH) is a syndrome of increased intracranial pressure characterized by normal brain imaging, normal cerebrospinal fluid (CSF) composition, and elevated CSF pressure. IIH occurs at the same rate in pregnant and nonpregnant women,105,108,113,114 but one study showed worsening of symptoms among nine of 11 patients with pre-existing IIH who became pregnant.106
The most common symptoms of IIH in pregnancy are headaches and visual changes. One study found that among 240 normal pregnant women, 12 developed IIH during one or more pregnancies. Ten had headaches, five had transient visual obscuration, four had reduced visual acuity, four had visual field loss, and three developed diplopia. All 12 women developed bilateral papilledema of varying severity.107
Visual outcome does not appear to differ between pregnant and nonpregnant women with IIH.105,107,108 A study of 13 pregnant IIH patients found that vision loss occurred at the same rate as age- and parity-matched controls.108 In addition, pregnancy outcomes are not adversely affected by IIH.105,109,110
The management of IIH during pregnancy includes weight control, nonketotic diet, steroids, and certain analgesics. Diuretics have been used in some cases. When medical management fails, serial lumbar punctures, optic nerve sheath fenestration, or lumboperitoneal shunt111 can be considered.105 Treatment decisions should be made in coordination with an obstetrician and possibly a neurosurgeon. Therapeutic abortion to limit progression of the disease generally is not indicated.105,111 The risk of recurrent IIH in subsequent pregnancies does not appear to be increased.109 Women who have developed IIH during pregnancy should not be advised against future pregnancies.112
Intracranial Aneurysms and Arteriovenous Malformations
Hemorrhagic cerebrovascular accidents (CVAs) are the most common neurosurgical diagnosis made in pregnancy.115 Intracranial hemorrhages are responsible for 4% to 12% of all maternal deaths133,134 and can be caused by arterial aneurysms, arteriovenous malformations (AVMs), metastatic choriocarcinoma, and disseminated intravascular coagulation (DIC).123,131 One retrospective study of 50,700 pregnancy-related admissions identified 13 hemorrhagic CVAs. Seven hemorrhages were subarachnoid and six were intracerebral. Five were caused by AVMs, three by arterial aneurysms, two by DIC, and three were idiopathic.131 Another retrospective meta-analysis study found that among 152 intracranial hemorrhages, 77% were caused by aneurysms and 23% by AVMs.134
Fifty percent of ruptured arterial aneurysms in women under the age of 40 are pregnancy-related.116 The hemodynamic and endocrinologic changes of pregnancy appear to be the cause of arterial alterations that can lead to new aneurysm formation or enlargement or rupture of a pre-existing aneurysm.115–118 Aneurysm rupture occurs more often in primiparous women115 and in the third trimester of pregnancy.115,129,131 In one study, 92% of intracranial hemorrhages occurred antepartum and 8% postpartum.134 In contrast, women with AVMs do not appear to be at a significantly increased risk of hemorrhage during pregnancy. Seven studies have reported no elevated risk of hemorrhage from AVMs during pregnancy.129,137–142
The most common presentation of a ruptured aneurysm or AVM is severe headache, seizure, or other neurologic signs from subarachnoid hemorrhage (SAH). However, enlarging aneurysms or AVMs can cause mass effects leading to cranial nerve palsies, optic nerve compression,119 or papilledema. One study reported that the possibility of focal neurologic deficit was higher among patients with intracranial AVMs than aneurysms.140 Patients with SAH can also develop subhyaloid or vitreous hemorrhage (Terson's syndrome).120 Secondary intraocular hemorrhage usually is managed conservatively,120 but vitrectomy may be indicated in bilateral or non-clearing cases.
The early diagnosis and management of a ruptured arterial aneurysm or AVM are critical in order to optimize the chances of maternal and fetal survival.116 The clinical challenge is that differentiating eclampsia from SAH can be difficult and may lead to a delay in diagnosis.116,118,121–123,134,135 The diagnosis is further complicated by the fact that pre-eclampsia and eclampsia are independent risk factors for aneurysm rupture124 so that these entities may coexist. Clinicians should maintain a high level of suspicion for intracranial aneurysm when pregnant women present with headache, seizure, altered consciousness, focal neurologic signs, visual changes, or diplopia. Urgent MRI and magnetic resonance angiography scanning is the current diagnostic modality of choice because there is no radiation risk or side effects of contrast media.132
The management of intracranial aneurysms generally is the same for pregnant as nonpregnant patients117,118 and neurosurgical considerations take precedence over obstetric considerations.117,137,144,145 When SAH occurs, endovascular obliteration,125,126,143 neurosurgical clipping,127 or stereotactic radiosurgery143 of the aneurysm or AVM may be carried out in order to avoid recurrent hemorrhage and secondary ischemia.115 One study of AVMs recommended that patients at high operative risk be managed conservatively until after delivery.143 Another study from 1990 reported that surgical management of aneurysms, but not AVMs, was associated with improved maternal and fetal survival. Taken together, these studies suggest that the threshold for neurosurgical intervention might be lower for aneurysms than AVMs, although this decision should be made by the neurosurgeon. The risk of rebleeding in the same pregnancy was 27% among AVMs in one study.136 Visual field and optic nerve changes can resolve over time following surgery.119,130
Once an aneurysm or AVM has been treated successfully, pregnancy can be allowed to progress to term. Most obstetricians prefer vaginal delivery in these cases,118,125 although one study reported a bias toward caesarian section among patients with AVM.143 Fetal mortality is not increased among mothers with intracranial aneurysms provided that the mother survives.128 Women with arterial aneurysms or AVMs are not advised against future pregnancy in most cases.129
Cerebral Venous and Sinus Thrombosis
Cerebral venous and sinus thrombosis (CVST) in the pregnant and puerperal state occurs with an incidence of 10 to 20 per 100,000 deliveries and accounts for 5% to 20% of all cerebral venous thrombosis.162 The signs and symptoms are nonspecific, which can lead to a potentially fatal delay in diagnosis.169,171 A retrospective study of 138 cases found that 74 (54%) presented in the first 7 days of the postpartum period.170 The most common signs and symptoms were fever (62%), headache (48%), seizure (46%), hemiplegia (43%), altered consciousness (41%), and papilledema (35%).170 Another retrospective study of 25 cases reported similar presenting clinical signs, which included headache (96%), focal neurologic deficits (60%), papilledema (43%), and seizure (40%).169 Magnetic resonance venography (MRV) is the preferred diagnostic modality, and treatment is with heparin anticoagulation.
The increased incidence of intracranial venous thrombosis is attributed to the hypercoagulable state of pregnancy,172,173 inherited protein C172,174,175 and S deficiencies,172,176,177 and eclampsia.178 A prospective study found that 78% of patients with venous thrombosis during pregnancy and the puerperium demonstrated activated protein C resistance (factor 5 Leiden).174 Three case reports have found associated protein S deficiency.172,176,177 There is one case report of transverse sinus thrombosis associated with eclampsia.178 The authors suggest a causative mechanism but this association may be incidental. It is recommended that causes of inherited hypercoagulability be investigated in all women who develop CVST during pregnancy or puerperium.
Mortality from CVST is less than 20%.171,179 The visual prognosis generally is good with blindness occurring because of late optic atrophy in two of 77 (3%) patients in one retrospective study.180 Women with pregnancy-related CVST should not be discouraged from future pregnancies.171,181 In one retrospective study, among 39 women with a history of CVST during pregnancy, 14 had 22 subsequent pregnancies resulting in 19 births and one elective and two spontaneous abortions. Five patients were prophylactically treated with low-dose heparin.181 In another study, among 77 women with pregnancy-related CVST there was no recurrence of CVST during subsequent pregnancies and only one postpartum deep venous thrombosis occurred.180
Metastatic Choriocarcinoma
Gestational choriocarcinoma is a malignant tumor that frequently undergoes hematogenous metastasis to highly vascularized organs such as the lung, brain, and liver. The incidence of choriocarcinoma is one in 50,000 live births and one in 30 molar pregnancies.146 Intracranial choriocarcinoma metastases can present as intracerebral,148–153 subarachnoid,153–155 or subdural hemorrhages.156,157 These tumors can bleed primarily or cause arterial aneurysms148,154,156,168 or arteriovenous fistulae155 with secondary bleeding. In a retrospective analysis of 10 cases, the most common presenting symptoms were features consistent with elevated intracranial pressure and hemiparesis.147 Ophthalmic signs and symptoms may be present and can include visual acuity or visual field loss, transient visual obscuration, papilledema, and retinal hemorrhages.147,154 It is important to consider this rare tumor in cases of young women presenting with stroke symptoms because it is potentially curable.149
Amniotic Fluid or Air Embolism
Amniotic fluid embolism is a rare but serious complication of pregnancy resulting in death in the majority of patients.160,161 Particulate matter of amniotic origin has been identified at autopsy in the brain, lungs, and kidneys.160 Focal neurologic deficits are rare162,164 but can affect the optic nerve, visual pathways, or occipital cortex. One case report describes isolated cortical blindness from air embolism that occurred following induced abortion and improved following treatment with hyperbaric oxygen.163
Carotid-Cavernous Fistulae
There are reports of five carotid-cavernous fistulas developing or worsening during pregnancy.165–168 One spontaneous fistula improved without intervention after the mother aborted the pregnancy in the twelfth week.165 A second spontaneous fistula worsened 3 weeks after a normal delivery before improving without intervention.165 One carotid-cavernous fistula was caused by metastatic choriocarcinoma.168 A traumatic fistula that had resolved 4 years prior to pregnancy recurred causing two antepartum and one postpartum hemorrhages. The authors propose that pregnancy-related hormonal and hemodynamic changes caused the recurrence and hemorrhage.167 However, the paucity of reports about carotid-cavernous fistulae during pregnancy suggest that this association may be incidental
OPTIC NERVE
Multiple Sclerosis and Optic Neuritis
Multiple sclerosis (MS) is a common cause of optic neuritis and is the most common chronic disabling neurologic disease affecting women of childbearing age.211 Pregnancy has a protective effect on MS with fewer and less severe relapses, especially during the third trimester.211–215 However, the relapse rate increases in the first 3 months following delivery before returning to pre-pregnancy rates.211–215 Breastfeeding and epidural analgesia have no deleterious effect on relapse rates.211,213,214 The Pregnancy and Multiple Sclerosis study prospectively followed 254 women with MS through 269 pregnancies. Compared with year prior to pregnancy, there was a reduction in the relapse rate during pregnancy, which was most marked in the third trimester. There was striking increase in the relapse rate in the first 3 months after delivery, although 72% of the women did not experience a relapse during these 3 months. After this, the rates returned to pre-pregnancy levels.213–214 Relapse rates remained at pre-pregnancy rates at the 2 year follow-up.214 A recent retrospective review of 351 women with MS confirmed the finding that the MS relapse rate decreases throughout pregnancy and increases during the puerperium.215
Overall, pregnancy has no long-term effect on MS related disability or disease course.211–214,216 Apart from methotrexate and cyclophosphamide, most drugs used routinely in the treatment of MS, including steroids, can be used safely by pregnant women.216 A study of 14 pregnant women with relapsing-remitting MS, who were treated with postpartum IVIg in an attempt to avoid postdelivery recurrences, found that none of the patients who received postpartum IVIg relapsed217 compared with the expected relapse rate of about 28%.214 Further study is needed to confirm this apparent protective effect.
Women with MS who desire children can be reassured that their infants are not at increased risk of malformations, preterm delivery, low birth weight, or infant death.211,213,214,216 Because a woman's past history of relapses may be the best indicator of future clinical course and long-term disability,212–214 women with more severe disease may wish to complete their families as soon as possible.216
A condition called “lactation optic neuritis” has been described in the first 12 months following delivery unassociated with known MS.204,205 The clinical course is similar to classic optic neuritis that occurs without lactation. It is possible that decreased immunosuppressive activity during the postpartum period induces the initial clinical manifestation of a previously unrecognized demyelinating disorder such as MS.204 The paucity of cases reported in the literature also suggests that this also could be an incidental association
ADDITIONAL PREGNANCY-RELATED OPTIC NEUROPATHIES
Devic's syndrome (neuromyelitis optica) is characterized by optic neuritis with transverse myelitis and may present during pregnancy.206,207 Severe vomiting in pregnancy (hyperemesis gravidarum) can lead to optic neuritis.208 One case of ischemic optic neuropathy has been reported in association with severe pre-eclampsia.209 A case of bilateral idiopathic optic neuritis with permanent visual loss recently was reported in association with pregnancy.210 One case of pregnancy-related papillophlebitis in a woman with no identifiable coagulation defect has been reported.349
OTHER NEURO-OPHTHALMOLOGIC DISORDERS
Cortical Vision Loss in Pre-Eclampsia and Eclampsia
Pre-eclampsia and eclampsia are the most common causes of pregnancy-related focal neurologic deficits. These deficits are characteristically of sudden onset and resolve over time. The precise pathogenesis of these strokelike focal deficits remains poorly understood.162,164 Transient visual changes occur in up to 25% of patients182 and cortical blindness is a rare but dramatic finding. A prospective study of 15 women who developed pre-eclampsia/eclampsia-related cortical blindness found complete resolution over 4 hours to 8 days.183 Another retrospective analysis of 19 cases of eclampsia reported transient cortical blindness in two patients with complete recovery in both.184
The etiology of cortical vision loss remains controversial. Many authors have proposed a mechanism of cerebral vasospasm with ischemic injury,183,185,186 whereas others suggest that increased capillary permeability leads to hydrostatic edema.187–189 Except for rare cases with persistent neurologic deficits and neuroradiologic abnormalities, the reversibility of vision loss argues against true ischemic necrosis.162 Recent studies have demonstrated cerebral vasospasm by angiography,185 transcranial color-coded sonography,185 and MRA188 with concurrent MRI,185,188 and single photon emission computed tomography187 findings consistent with cerebral edema. This suggests that cerebral vasospasm leads to relative ischemia and changes in regional blood flow that cause reversible edema. Hypercalcemia190 and inflammatory cytokines of placental origin191 recently have been proposed as putative inciting factors.
Horner's Syndrome
Horner's syndrome has been reported as a complication of epidural anesthesia for labor. Although not uncommon in women in labor, this complication is virtually unknown in patients outside of the context of labor.218 The incidence of Horner's syndrome was estimated in a prospective study of 200 women who received epidural analgesia during labor. The incidence was 1.3% among 150 who delivered vaginally and 4% among 50 patients who underwent caesarian section.221 There are two case reports of trigeminal nerve palsy following epidural anesthesia for labor.219,223
The etiology seems to be cephalad spread of local anesthesia, sometimes via unexplained routes and with surprising selective targeting effect.219 The propensity in pregnant women is believed to be caused by pregnancy-related anatomic and physiologic changes of the epidural space.219 In one case report, an epidurogram after Horner's syndrome developed from epidural anesthesia demonstrated unilateral left spread ascending to upper thoracic levels with delayed spread toward the right lumbar epidural space.220 This complication generally is transient in nature but must be accompanied by close maternal and fetal monitoring because it can be associated with maternal hypotension caused by autonomic effects.222
Myasthenia Gravis
Myasthenia gravis (MG) frequently affects young women in their childbearing years. Several retrospective studies suggest that approximately 40% of women with pre-existing MG clinically worsen during pregnancy.224–229 This deterioration usually occurs during the first trimester226,229 or shortly after delivery.224,225 The authors are not aware of any studies that specifically evaluate the ophthalmic signs and symptoms of MG during pregnancy but suspect that they would approximate overall disease activity.
The rate of neonatal myasthenia gravis (NMG) after delivery varies widely in the literature from 6% to 50%.225,228–231 The incidence of NMG seems to be more common among infants born to mothers with puerperal infections or shorter antenatal disease duration.224 The newborns of thymectomized mothers are relatively protected.224 Two studies demonstrate good correlation between maternal antiacetylcholine receptor antibody titers and the development of NMG, so this testing may be helpful.228,231 The largest study of neonatal outcomes retrospectively evaluated 127 births of mothers with MG compared with population-wide controls. Women with MG had 8% more complications during delivery, premature rupture of membranes was three times more common, and caesarian sections were twice as common. Five children (3.9%) had severe anomalies and three of them died.232 Another study showed that perinatal mortality also was increased among mothers who remained asymptomatic during pregnancy.233 This suggests that neonatal outcomes are not only related to MG disease severity but also to effects of the underlying immunologic dysfunction on the fetus. To the authors' knowledge, perinatal mortality has not been evaluated for cases of isolated ocular myasthenia.
Treatment of MG during pregnancy requires close coordination among the obstetrician, neurologist, pediatrician, and ophthalmologist when ocular symptoms are present. Treatment during pregnancy may include immunosuppressive therapy, plasmapheresis, intravenous human immunoglobulins,225 and thymectomy.234
Ocular Migraine
The lifetime prevalence of migraine with aura (MA) is 5%, with a female to male ratio of 2 to 1.235 Visual disturbances are the most common aura phenomenon, occurring in 90% of patients with MA.235 Several retrospective and two prospective studies have demonstrated improvement of migraine during pregnancy.236–240 The largest prospective study included 49 migraineurs who became pregnant. Migraine symptoms improved in 47% during the first trimester, 85% during the second trimester, and 87% in the third trimester. Complete remission was achieved in 79% of women by the end of pregnancy. Migraine recurred in 55% during the first month after delivery but breastfeeding seemed to afford some protection.237 Retrospective studies have demonstrated that women with migraine onset at menarche and those with perimenstrual migraine are more likely to go into remission during pregnancy.238 However, this association has not been observed in prospective studies.236,237 Retrospective studies also have shown that migraine with aura appears to be overrepresented among the small number of women (4%–8%) whose migraines worsen during pregnancy.238 Migraine can begin during pregnancy in 1% to 17% of cases, often during the first trimester, and involves a higher proportion with aura.238 The positive effects of pregnancy on migraine probably are related to uniformly high and stable estrogen levels.240,241
The symptoms of headache and visual changes during pregnancy are nonspecific. The development or worsening of migrainelike symptoms during pregnancy can indicate an underlying structural lesion or disease process such as an arterial aneurysm, arteriovenous malformation, progressive meningioma, venous sinus thrombosis, or pre-eclampsia, all of which are more common during pregnancy. Therefore, appropriate diagnostic testing should be performed to rule out other organic causes of headache and visual changes.242,243
Migraine during pregnancy does not increase the risk of pregnancy-related complications such as pre-eclampsia/eclampsia, abnormal labor, fetal loss, or congenital anomalies.238 Safe and effective acute care treatments include paracetamol, opioids, and antiemetics.244 The use of triptans appears to be safe, but data are limited and they are not broadly recommended.244,245 Preventative treatments include relaxation, biofeedback, beta-blockers such as labetalol, some antidepressants, and gabapentin.244,246