1. Anteby I, Cohen E, Anteby E, et al: Ocular manifestations in children born after in vitro fertilization. Arch Ophthalmol 119:1525, 2001 2. Sunness JS: The pregnant woman's eye. Surv Ophthalmol 32:219, 1988 3. Hørven I, Gjonnaess H: Corneal indentation pulse and intraocular pressure in pregnancy. Arch Ophthalmol 91:92, 1974 4. Myer EJ, Roberts CR, Leibowitz HM, et al: Influence of norethynodrel with mestranol on intraocular pressure in glaucoma: A
controlled double-blind study. Arch Ophthalmol 75:771, 1966 5. Kass MA, Sears ML: Hormonal regulation of intraocular pressure. Surv Ophthalmol 22:153, 1977 6. Phillips CI, Gore SM: Ocular hypotensive effect of late pregnancy with and without high blood
pressure. Br J Ophthalmol 69:117, 1985 7. Millodot M: The influence of pregnancy on the sensitivity of the cornea. Br J Ophthalmol 61:646, 1977 8. Weinreb RN, Lu A, Beeson C: Maternal corneal thickness during pregnancy. Am J Ophthalmol 105:258, 1988 9. Duncan TE: Krukenberg spindles in pregnancy. Arch Ophthalmol 91:355, 1974 10. Frenkel M, Klein HZ: Malignant melanoma of the choroid in pregnancy. Am J Ophthalmol 62:910, 1966 11. Seddon JM, MacLaughlin DT, Albert DM, et al: Uveal melanomas presenting during pregnancy and the investigation of oestrogen
receptors in melanomas. Br J Ophthalmol 66:695, 1982 12. Fastenberg DM, Ober RR: Central serous choroidopathy in pregnancy. Arch Ophthalmol 101:1055, 1983 13. Chang M, Herbert WNP: Retinal arteriolar occlusions following amniotic fluid embolism. Ophthalmology 91:1634, 1984 14. Sanke RF: Blepharoptosis as a complication of pregnancy. Ann Ophthalmol 16:720, 1984 15. Duke-Elder S: System of Ophthalmology, Vol. 13, p. 857. St. Louis, CV Mosby, 1974 16. Digre KB, Varner MW, Corbett JJ: Pseudotumor cerebri and pregnancy. Neurology 34:721, 1984 17. Kenny GS, Cerasoli JR: Color fluorescein angiography in toxemia of pregnancy. Arch Ophthalmol 87:383, 1972 18. Jaffe G, Schatz H: Ocular manifestations of preeclampsia. Am J Ophthalmol 103:309, 1987 19. Beck RW, Gamel JW, Willcourt RJ, et al: Acute ischemic optic neuropathy in severe preeclampsia. Am J Ophthalmol 90:342, 1980 20. Duncan R, Hadley D, Bone I, et al: Blindness in eclampsia: CT and MR imaging. J Neurol Neurosurg Psychiatry 52:899, 1989 21. Sadovsky A, Serr DM, Landou J: Retinal changes and fetal prognosis in toxemias of pregnancy. Obstet Gynecol 8:426, 1956 22. Sihota RS, Bose S, Paul AH: The neonatal fundus in maternal toxemia. J Pediatr Ophthalmol Strabismus 26:281, 1989 23. Oliver M, Uchenik D: Bilateral exudative retinal detachment in eclampsia without hypertensive
retinopathy. Am J Ophthalmol 90:792, 1980 24. Gitter KA, Hauser BP, Sarin LK, et al: Toxemia of pregnancy: An angiographic interpretation of fundus changes. Arch Ophthalmol 80:449, 1968 25. Chaine G, Attali P, Gaudric A, et al: Ocular fluorophotometric and angiographic findings in toxemia of pregnancy. Arch Ophthalmol 104:1632, 1986 26. Folk JC, Weingeist TA: Fundus changes in toxemia. Ophthalmology 88:1173, 1981 27. Phelps RL, Sakol P, Metzger B, et al: Changes in diabetic retinopathy during pregnancy correlations with regulation
of hyperglycemia. Arch Ophthalmol 104:1806, 1986 28. Johnston GP: Pregnancy and diabetic retinopathy. Am J Ophthalmol 90:519, 1980 29. Samples JR, Meyer SM: Use of ophthalmic medications in pregnant and nursing women. Am J Ophthalmol 106: 616, 1988 30. Shepard TH: Teratogenicity of therapeutic agents. Curr Probl Pediatr 10:1, 1979 31. Fraser FC: Relation of animal studies to the problem in man. In Wilson JG, Fraser FC (eds): Handbook of Teratology, Vol. 1, p. 75. New York, Plenum, 1977 32. Kalter H, Warkany J: Congenital malformations. N Engl J Med 308:424, 1983 33. Rudolph RS: Vital statistics: U.S. In Rudolph AM (ed): Pediatrics, 18th ed, p. 6. Norwalk, Appleton & Lange, 1987 34. Wilson JG: Current status of teratology: General principles and mechanisms derived
from animal studies. In Wilson JG, Fraser FC (eds): Handbook of Teratology, Vol. I, p. 47. New York, Plenum, 1977 35. Duke-Elder S, Cook C: Normal development. Part 1. Embryology. In Duke-Elder S (ed): System of Ophthalmology, Vol. 3, p. 29. St. Louis, CV Mosby, 1963 36. Rudolph AJ, Desmond MM: Clinical manifestations of the congenital rubella syndrome. Int Ophthalmol Clin 12:3, 1972 37. Hardy JB, McCracken GH Jr, Gilkeson MR, et al: Adverse fetal outcome following maternal rubella after the first trimester
of pregnancy. JAMA 207:2414, 1969 38. Gillette JR: Factors that affect drug concentrations in maternal plasma. In Wilsonm JG, Fraser FC (eds): Handbook of Teratology, Vol. 2, p. 35. New York, Plenum, 1977 39. Wilson JG: Embryological considerations in teratology. Ann NY Acad Sci 123:219, 1965 40. Wilson JG: An animal model of human disease: Thalidomide embryopathy in primates. Comp Pathol Bull 5:3, 1973 41. Stromland K, Miller M, Cook C: Ocular teratology. Surv Ophthalmol 35:429, 1991 42. Heinonen OP, Slone D, Shapiro S: Birth Defects and Drugs in Pregnancy. Littleton, MA, Publishing Sciences Group, 1977 43. Shepard TH: Catalog of Teratogenic Agents, 6th ed. Baltimore, Johns Hopkins University Press, 1989 44. Schardein JL: Drugs as Teratogens. Cleveland, CRC Press, 1976 45. Wilson JG, Fraser FC (eds): Handbook of Teratology. New York, Plenum, 1977 46. Weinstein L (ed): Teratology and Congenital Malformations: A Comprehensive Guide to the Literature. New York, IFI/Plenum, 1976 47. Briggs GG, Freeman RK, Yaffe SJ: Drugs in Pregnancy and Lactation, 2nd ed. Baltimore, Williams & Wilkins, 1986 48. Brent RL, Beckman DA: Environmental Teratogens. Bull NY Acad Med 66:123, 1990 49. Apt L, Gaffney WL: Congenital eye abnormalities from drugs during pregnancy. In Leopold I (ed): Symposium of Ocular Therapy, p. 1. St. Louis, CV Mosby, 1974 50. Spaeth GL, Nelson LB, Beaudoin AR: Ocular teratology. In Tasman W, Jaeger EA (eds): Duane's Biomedical Foundations of Ophthalmology, Vol. 1, p. 2. Philadelphia, JB Lippincott, 1989 51. Palmer EA: Teratogenic agents. In Isenberg SJ (ed): The Eye in Infancy, p. 110. Chicago, Year Book Medical Publishers, 1989 52. Ullberg S, Lindquist NG, Sjostrand SE: Accumulation of chorioretinotoxic drugs in the fetal eye. Nature 227:1257, 1970 53. Dencker L, Lindquist NG, Ullberg S: Distribution of an I-125 labeled chloroquine analogue in a pregnant
macaca monkey. Toxicology 5:255, 1975 54. Hart CW, Nautan RF: The ototoxicity of chloroquine phosphate. Arch Otolaryngol 80:407, 1964 55. Smith DW: Dysmorphology (teratology). J Pediatr 69: 1150, 1966 56. Winckel CFW: Quinine and congenital injuries of the ear and eye of the fetus. J Trop Med Hyg 51:2, 1948 57. Reed H, Briggs JN, Martin JK: Congenital glaucoma, deafness, mental deficiencies and cardiac anomaly
following attempted abortion. J Pediatr 46:182, 1955 58. Richardson S: The toxic effect of quinine on the eye. South Med J 29:1156, 1936 59. Slone D, Siskind V, Heinonen OP, et al: Antenatal exposure to the phenothiazines in relation to congenital malformations, prenatal
mortality rate, birth weight and intelligence quotient
score. Am J Obstet Gynecol 128:486, 1977 60. Rumeau-Rouquette C, Goujard J, Huel G: Possible teratogenic effect of phenothiazines in human beings. Teratology 15:57, 1977 61. Milkovich L, den Berg BJ: An evaluation of the teratogenicity of certain antinauseant drugs. Am J Obstet Gynecol 125:244, 1976 62. Fairgrieve SD, Jackson M, Jonas P, et al: Population based, prospective study of the care of women with epilepsy
in pregnancy. BMJ 321:674-5, 2000 63. Hanson JW, Myrianthopoulos NC, Sedgwick Harvey MA, et al: Risks to offspring of women treated with hydantoin anticonvulsants, with
emphasis on the fetal hydantoin syndrome. J Pediatr 89:662, 1976 64. Hanson JW: Teratogen update: Fetal hydantoin effects. Teratology 33:349, 1986 65. Hanson JW, Smith DW: The fetal hydantoin syndrome. J Pediatr 87:285, 1975 66. Smith DW: Teratogenicity of anticonvulsive medications. Am J Dis Child 131:1337, 1977 67. Bartoshesky LE, Bhan I, Nagpaul K, et al: Severe cardiac and ophthalmologic malformations in an infant exposed to
diphenylhydantoin in utero. Pediatrics 69:202, 1982 68. Hoyt CS, Billson FA: Maternal anticonvulsants and optic nerve hypoplasia. Br J Ophthalmol 62:3, 1978 69. Wallar PH, Genstler DE, George CC: Multiple systemic and periocular malformations associated with the fetal
hydantoin syndrome. Ann Ophthalmol 10:1568, 1978 70. Wilson RS, Smead W, Char F: Diphenylhydantoin teratogenicity: Ocular manifestations and related deformities. J Pediatr Ophthalmol Strabismus 15:137, 1978 71. Hampton GR, Krepostman JI: Ocular manifestations of the fetal hydantoin syndrome. Clin Pediatr (Phila) 20:475, 1981 72. Tunnessen WW Jr, Lowenstein EH: Glaucoma associated with the fetal hydantoin syndrome. J Pediatr 89:154, 1976 73. Dabee V, Hart AG, Hurley RM: Teratogenic effects of diphenylhydantoin. Can Med Assoc J 112:75, 1975 74. Gaily E, Kantola-Sorsa E, Granström M-L: Intelligence of children of epileptic mothers. J Pediatr 113:677, 1988 75. Van Dyke DC, Hodge SE, Heide F, et al: Family studies in fetal phenytoin exposure. J Pediatr 113:301, 1988 76. Allen RW: Fetal hydantoin syndrome and malignancy. J Pediatr 105:681, 1984 77. Zackai EH, Mellman WJ, Neiderer B, et al: The fetal trimethadione syndrome. J Pediatr 87:280, 1975 78. Feldman GL, Weaver DD, Lourien EW: The fetal trimethadione syndrome: Report of an additional family and further
delineation of this syndrome. Am J Dis Child 131:1389, 1977 79. Krauss CM, Holmes LB, Van Lang QCN, et al: Four siblings with similar malformations after exposure to phenytoin and
primidone. J Pediatr 105:750, 1984 80. Jones KL, Lacro RV, Johnson KA, et al: Pattern of malformations in the children of women treated with carba-mazepine
during pregnancy. N Engl J Med 320:1661, 1989 81. Ardinger HH, Atkin JF, Blackston D, et al: Verification of the fetal valproate syndrome phenotype. Am J Med Genet 29:171, 1988 82. Buehler BA, Delimont D, van Waes M, et al: Prenatal prediction of risk of the fetal hydantoin syndrome. N Engl J Med 322:1567, 1990 83. Smith DB, Carl GF: Interactions between folates and caramazepine or valproate in the rat. Neurology 32:965, 1982 84. Dansky LV, Rosenblatt DS, Andermann E: Mechanisms of teratogenesis: folic acid and antiepiletic therapy. Neurology 42:32, 1992 85. Kerber U, Warr OSIII , Richardson C: Pregnancy in a patient with prosthetic mitral valve: Associated with a
fetal anomaly attributed to warfarin sodium. JAMA 203:223, 1968 86. Shaul WL, Emery H, Hall JG: Chondrodysplasia punctata and maternal warfarin during pregnancy. Am J Dis Child 129:360, 1975 87. Pauli RM, Madden JD, Kranzler KJ, et al: Warfarin therapy initiated during pregnancy and phenotypic chondrodysplasia
punctata. J Pediatr 88:506, 1976 88. Becker MH, Genieser NB, Finegold M, et al: Chondrodysplasia punctata: Is warfarin therapy a factor?Am J Dis Child 129:356, 1975 89. Warkany J: Warfarin embryopathy. Teratology 14:205, 1976 90. Stevenson RE, Burton OM, Ferlauto GJ: Hazards of oral anticoagulants during pregnancy. JAMA 243:1549, 1980 91. Hall JG, Pauli RM, Wilson KM: Maternal and fetal sequelae of anticoagulants during pregnancy. Am J Med 68: 122, 1980 92. Harrod MJE, Sherrod PS: Warfarin embryopathy in siblings. Obstet Gynecol 57:673, 1981 93. Kaplan LC: Congenital Dandy Walker malformation associated with first trimester. Warfarin: A
case report and literature review. Teratology 32:333, 1985 94. Nicholson HO: Cytotoxic drugs in pregnancy. J Obstet Gynecol Br Commonw 75:307, 1968 95. Chernoff N, Rogers JM, Alles AJ, et al: Cell cycle alternations and cell death in cyclophosphamide teratogenesis: Teratogenesis Carcinog Mutagen 9:199, 1989 96. Smith SB, Cooke CB, Yielding KL: Effects of the antioxidant butylated hydroxytoluene (BHT) on
retinal degeneration induced transplacentally by a single low dosage of N-methyl-N-nitrosourea (MNV). Teratogenesis Carcinog Mutagen 8:175, 1988 97. Narbaitz R, Marino I: Experimental induction of microphthalmia in the chick embryo with a single
dose of cisplatin. Teratology, 37:127, 1988 98. Diamond I, Anderson MM, McCreadies SR: Transplacental transmission of busulfan (Myleran) in mother with
leukemia (production of fetal malformation and cytomegaly). Pediatrics 25:85, 1960 99. Pruitt AW, Jacobs EA, Schydlower M, et al: Committee on substance abuse: Drug-exposed infants. Pediatrics 86: 639, 1990 100. Chasnoff IJ, Burns WJ, Schnoll SH, et al: Cocaine use in pregnancy. N Engl J Med 313:666, 1985 101. Chasnoff IJ, Bussey ME, Savich R, et al: Perinatal cerebral infarction and maternal cocaine use. J Pediatr 108: 456, 1986 102. Isenberg SJ, Spierer A, Inkelis SH: Ocular signs of cocaine intoxication in neonates. Am J Ophthalmol 103:211, 1987 103. Teske MP, Trese MT: Retinopathy of prematurity-like fundus and persistent hyperplastic
primary vitreous associated with maternal cocaine use. Am J Ophthalmol 103: 719, 1987 104. Laegreid L, Olegard R, Walstrom J, et al: Teratogenic effects of benzodiazepine use during pregnancy. J Pediatr 114:126, 1989 105. Long SY: Does LSD induce chromosome damage and malformations? A review of the literature. Teratology 6:75, 1972 106. Hanaway SK: LSD, effects on the developing mouse lens. Science 164:574, 1969 107. Apple DJ, Bennett TO: Multiple systemic and ocular malformations associated with maternal LSD
usage. Arch Ophthalmol 92:301, 1974 108. Chan CC, Fishman M, Egbert PR: Multiple ocular anomalies associated with maternal LSD ingestion. Arch Ophthalmol 96:282, 1978 109. Margolis S, Martin L: Anophthalmia in an infant of parents using LSD. Ann Ophthalmol 12:1378, 1980 110. Pleet H, Graham JM, Smith DW: Central nervous system and facial defects associated with maternal hyperthermia
at four to fourteen weeks' gestation. Pediatrics 67:785, 1981 111. Warkany J: Teratogen update: Hyperthermia. Teratology 33:365, 1986 112. Graham JM, Edwards MJ, Lipson WS, et al: Gestational hyperthermia as a cause for Möebius syndrome. Teratology 37:461, 1988 113. Rosa FW, Wilk AL, Kelsey FO: Teratogen update: Vitamin A congeners. Teratology 33:355, 1986 114. Cook CS, Sulik KK: Laminin and fibronectin in retinoid-induced keratolenticular dysgenesis. Invest Ophthalmol Vis Sci 31:751, 1990 115. Peck GL, Olsen TG, Yoder FW, et al: Prolonged remissions of cystic and conglobate acne with 13-cis-retinoic
acid. N Engl J Med 300:329, 1979 116. Lammer EJ, Chen DT, Hoar RM, et al: Retinoic acid embryopathy. N Engl J Med 313:837, 1985 117. Fernhoff PM, Lammer EJ: Craniofacial features of isotretinoin embryopathy. J Pediatr 105:595, 1984 118. Lott IT, Bocian M, Pribram HW, et al: Fetal hydrocephalus and ear anomalies associated with maternal use of isotretinoin. J Pediatr 105:597, 1984 119. Hall JG: Vitamin A: A newly recognized human teratogen. Harbinger of things to come. J Pediatr 105:583, 1984 120. De La Cruz E, Sun S, Vangvanichyakorn K, et al: Multiple congenital anomalies associated with maternal isotretinoin therapy. Pediatrics 74:428, 1984 121. Lammer EJ: Embryopathy in infant conceived one year after termination of maternal
etretinate. Lancet 2:1080, 1988 122. Lenz W: Thalidomide and congenital abnormalities. Lancet 1:45, 1962 123. McBride WG: Thalidomide and congenital abnormalities. Lancet 2:1358, 1961 124. Miller MT, Stromland K: Teratogen update: thalidomide: A review, with a focus on ocular findings
and new potential uses. Teratology 60:306, 1999 125. Thiersch JB, Phillips FS: Effect of 4-amino-pteroylglutamic acid (aminopterin) on
early pregnancy. Proc Soc Exp Biol Med 74:204, 1950 126. Thiersch JB: Therapeutic abortions with a folic acid antagonist, 4-aminopteroylglutamic
acid (4-amino PGA) administered by the oral
route. Am J Obstet Gynecol 63:1298, 1952 127. Warkany J, Beauadry PH, Hornstein S: Attempted abortion with 4-aminopteroglutamic acid (aminopterin): Malformation
of the child. Am J Dis Child 97:274, 1959 128. Emerson DJ: Congenital malformations due to attempted abortion with aminopterin. Am J Obstet Gynecol 84:356, 1962 129. Shaw EB: Fetal damage due to maternal aminopterin ingestion: Follow-up at
age 9 years. Am J Dis Child 124:93, 1972 130. Milunsky A, Graef JW, Gaynor MF: Methotrexate-induced congenital malformations. J Pediatr 72:790, 1968 131. Darab DJ, Minkoff R, Sciote J, et al: Pathogenesis of median facial clefts in mice treated with methotrexate. Teratology 36:77, 1987 132. Kurland LT, Faro SN, Siedler H: Minamata disease. World Neurol 1:370, 1960 133. Matsumoto H, Koya G, Takeuchi T: Fetal Minamata disease. J Neuropathol Exp Neurol 24:563, 1965 134. Curley A, Sedlak VA, Girling EF, et al: Organic mercury identified as a cause of poisoning in humans and hogs. Science 172:65, 1971 135. Harada M: Congenital Minamata disease: Intrauterine methylmercury poisoning. Teratology 18:285, 1978 136. Amin-Zaki L, Elhassani S, Majeed MA, et al: Intra-uterine methylmercury poisoning in Iraq. Pediatrics 54:587, 1974 137. Snyder RD: Congenital mercury poisoning. N Engl J Med 284:1014, 1971 138. Koos BJ, Longo LD: Mercury toxicity in the pregnant woman, fetus, and newborn infant. A review. Am J Obstet Gynecol 126:390, 1976 139. Amin-Zaki L, Majeed MA, Elhassani SB, et al: Prenatal methylmercury poisoning. Am J Dis Child 133:172, 1979 140. Gasset AR, Itoi M, Ishii Y, et al: Teratogenicities of ophthalmic drugs. Part II. Teratogenicities and tissue
accumulation of thimerosal. Arch Ophthalmol 93:52, 1975 141. Lamache MA: Communications: Reflections sur la déscendance des alcooliques. Bull Nat Med 151:517, 1967 142. Lemoine P, Harousseau H, Borteyru JP, et al: Les enfants de parents alcooliques: Anomalies observées: A propos
de 127 cas. Quest Med 25:475, 1968 143. Hanson JW, Jones KL, Smith DW: Fetal alcohol syndrome: Experience with 41 patients. JAMA 235:1458, 1976 144. Shaywitz SE, Cohen DJ, Shaywitz BA: Behavior and learning difficulties in children of normal intelligence born
to alcoholic mothers. J Pediatr 96:978, 1980 145. Stromland K: Ocular involvement in the fetal alcohol syndrome. Surv Ophthalmol 31:277, 1987 146. Graham JM: Independent dysmorphology evaluations at birth and 4 years of age for children
exposed to varying amounts of alcohol in utero. Pediatrics 81:772, 1988 147. Karl PI, Gordon BHJ, Lieber CS, et al: Acetaldehyde production and transfer by the perfused human placental cotyledon. Science 242:273, 1988 148. Jones KL, Hanson JW, Smith DW: Palpebral fissure size in newborn infants. J Pediatr 92:787, 1978 149. Fuch M, Iosub S, Bingol N, et al: Palpebral fissure size revisited. J Pediatr 96:77, 1980 150. Miller M, Israel J, Cuttone J: Fetal alcohol syndrome. J Pediatr Ophthalmol Strabismus 18:6, 1981 151. Miller MT, Epstein RJ, Sugar J, et al: Anterior segment anomalies associated with the fetal alcohol syndrome. J Pediatr Ophthalmol Strabismus 21:8, 1984 152. Stromland K, Hellstrom A: Fetal alcohol syndrome: An opthalmological and socioeducational perspective
study. Pediatrics 97:845, 1996 153. Cook CS, Nowotny AZ, Sulik KK: Fetal alcohol syndrome: Eye malformations in a mouse model. Arch Ophthalmol 105:1576, 1987 154. Brent RL: Radiation teratogenesis. Teratology 21:281, 1980 155. Rugh R, Skaredoff L: X-rays and the monkey fetal retina. Invest Ophthalmol 8:31, 1969 156. Miller RW: Effects of ionizing radiation from the atomic bomb on Japanese children. Pediatrics 41:257, 1968 157. Dekaban AS: Abnormalities in children exposed to x-radiation during various
stages of gestation: Tentative timetable of radiation injury to the human
fetus. Part I. J Nucl Med 9:471, 1968 158. Greim MD, Meier P, Dobben GD: Analysis of the morbidity and mortality of children irradiated in fetal
life. Radiology 88:347, 1967 159. Jacobsen L, Mellemgaard L: Anomalies of the eyes in descendants of women irradiated with small x-ray
doses during age of fertility. Acta Ophthalmol 46:352, 1968 160. Marion RW, Wiznia AA, Hutcheon RG, et al: AIDS embryopathy: A new dysmorphic syndrome in children with the acquired
immunodeficiency syndrome. Am J Dis Child 140:638, 1986 161. Itoi M, Gefter JW, Kaneko N, et al: Teratogenicities of ophthalmic drugs. Part 1. Antiviral ophthalmic drugs. Arch Ophthalmol 93:46, 1975 162. Ballard PD, Hearnery EF, Smith MB: Induction of cleft palate in mice after ophthalmic administration of hydrocortisone. Toxicol Appl Pharmacol 34:358, 1975 163. Baden E: Environmental pathology of the teeth. In Gorlin RJ, Goldman HH (eds): Thomas' Oral Pathology, 6th ed. St. Louis, CV Mosby, 1970 164. Sullivan G, Takacs E: Comparative teratogenicity of pyrimethamine in rats and hamsters. Teratology 4:250, 1971 165. Sand BJ, Shanedling PD: Teratogenesis from Daraprim. Am J Ophthalmol 56:1011, 1963 166. Rogoyski A, Trzcinska-Dabrowska Z: Corticosteroid-induced cataract and palatoschisis in the mouse fetus. Am J Ophthalmol 68:128, 1969 167. Kasirsky G, Lombardi L: Comparative teratogenic study of various corticoid ophthalmics. Toxicol Appl Pharmacol 16:773, 1970 168. Holmes LB, Trelstad RL: The early limb deformity caused by acetazolamide. Teratology 20:289, 1979 169. Isenberg SJ: Ocular trauma. In Isenberg SJ (ed): The Eye in Infancy, p. 377. Chicago, Yearbook Medical Publishers, 1989 170. Sachs D, Levin PS, Dooley K: Marginal eyelid laceration at birth. Am J Ophthalmol 102:539, 1986 171. Harris GJ: Canalicular laceration at birth. Am J Ophthalmol 105:322, 1988 172. Isenberg SJ, Heckenlively JR: Traumatized eye with retinal damage from amniocentesis. J Pediatr Ophthalmol Strabismus 22:65, 1985 173. Thomas G, Blackwell RJ: A hazard associated with the use of spiral fetal scalp electrodes. Am J Obstet Gynecol 121:1118, 1975 174. Duke-Elder S, Mac Paul PA: Injuries, Part 1, Mechanical Injuries. In Duke-Elder S (ed): System of Ophthalmology, Vol. 14, p. 9. St. Louis, CV Mosby, 1972 175. Dutescu M, Lappe A: Luxation of the eyeball in the newborn. J Pediatr Ophthalmol 11:82, 1974 176. Hofmann RF, Paul TO, Pentelei-Molnar J: The management of corneal birth trauma. J Pediatr Ophthalmol Strabismus 18:45, 1981 177. Angell LK, Robb RM, Berson FG: Visual prognosis in patients with ruptures in Descemet's membrane
due to forceps injuries. Arch Ophthalmol 99:2137, 1981 178. Stein RM, Cohen EJ, Calhoun JH, et al: Corneal birth trauma managed with a contact lens. Am J Ophthalmol 103:596, 1987 179. Spencer WH, Ferguson WJ, Shaffer RN, et al: Late degenerative changes in the cornea following breaks in Descemet's
membrane. Trans Am Acad Ophthalmol Otolaryngol 70:973, 1966 180. Wu G, Behrens MM: Hyphema in the newborn: Report of a case. J Pediatr Ophthalmol Strabismus 19:56, 1982 181. Pulido JS, Lingua RW, Cristol S, et al: Protein C deficiency associated with vitreous hemorrhage in a neonate. Am J Ophthalmol 104:546, 1987 182. Duke-Elder S, Dobree JH: Diseases of the retina. In Duke-Elder S (ed): System of Ophthalmology, Vol. 10, p. 139. St. Louis, CV Mosby, 1967 183. Levin S, Janive J, Mintz M, et al: Diagnostic and prognostic value of retinal hemorrhages in the neonate. Obstet Gynecol 55:309, 1980 184. Chace RR, Merritt KK, Bellows M: Ocular findings in the newborn infant. Arch Ophthalmol 44:236, 1950 185. Von Barsewich B: Perinatal Retinal Hemorrhages. New York, Springer-Verlag, 1979 186. Rosenbaum AL, Phelps DL, Isenberg SJ, et al: Retinal hemorrhages in retinopathy of prematurity associated with tocopherol
treatment. Ophthalmology 92:1012, 1985 187. Russell PA: Subdural haematoma in infancy. BMJ 2:446, 1965 188. Emerson MV, Pieramici DJ, Stoessel KM, et al: Incidence and rate of disappearance of retinal hemorrhage in newborns. Opthalmology. 108:36, 2001 189. Volpe JJ: Injuries of extracranial, cranial, intracranial, spinal cord, and peripheral
nervous system structures. In VolpeJJ: Neurology of the Newborn, p. 638. Philadelphia, WB Saunders, 1987 190. Walsh TJ, Smith JL, Shipley T: Blindness in infants. Am J Ophthalmol 62:546, 1966 191. Falco NA, Eriksson E: Facial nerve palsy in the newborn: Incidence and outcome. Plast Reconstr Surg 85:1, 1990 192. Eng GD: Neuromuscular diseases. In Avery GB (ed): Neonatology: Pathophysiology and Management of the Newborn, 3rd ed, p. 1158. Philadelphia, JB Lippincott, 1987 193. Jaffe N, Cassady R, Peterson R, et al: Heterochromia and Horner syndrome associated with cervical and mediastinal
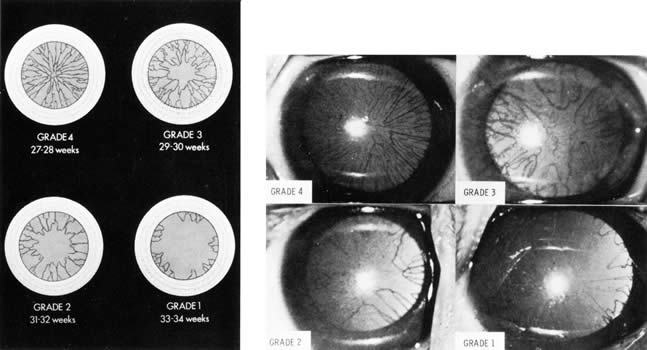
neuroblastoma. J Pediatr 87:75, 1975 194. Reisner SH, Perlman M, Ben-Tovim N, et al: Transient lateral rectus muscle paresis in the newborn infant. J Pediatr 78:461, 1971 195. Crawford JS: Ptosis as a result of trauma. Can J Ophthalmol 9:244, 1974 196. Blechman B, Isenberg S: An anatomical etiology of congenital eyelid eversion. Ophthalmic Surg 15:111, 1984 197. Dubowitz LMS, Dubowitz V, Goldberg C: Clinical assessment of gestational age in the newborn infant. J Pediatr 77:1, 1970 198. Ballard JL, Novak KK, Driver M: A simplified score for assessment of fetal maturation of newly born infants. J Pediatr 95:769, 1979 199. Hittner HM, Hirsch NJ, Rudolph AJ: Assessment of gestational age by examination of the anterior vascular capsule
of the lens. J Pediatr 91:455, 1977 200. Hittner HM, German WA, Rudolph AJ: Examination of the anterior vascular capsule of the lens, Part II. Assessment
of gestational age in infants small for gestational age. J Pediatr Ophthalmol Strabismus 18:52, 1981 201. Mimouni F, Nissenkorn I, Wilunski E, et al: Assessment of gestational age by examination of the anterior vascular capsule
of the lens: Value in multiple pregnancy (quintuplets). J Pediatr Ophthalmol Strabismus 20:27, 1983 202. Hittner HM, Speer ME, Rudolph AJ: Examination of the anterior vascular capsule of the lens. Part III. Abnormalities
in infants with congenital infection. J Pediatr Ophthalmol Strabismus 18:55, 1981 203. Hiles DA, Wallar PH, MacFarlane F: Current concepts in the management of strabismus in children with cerebral
palsy. Ann Ophthalmol 7:789, 1975 204. Cats BP, Tan KEWP: Prematures with and without regressed retinopathy of prematurity: Comparison
of long-term (6–10 years) ophthalmological
morbidity. J Pediatr Ophthalmol Strabismus 26:271, 1989 205. Alden ER, Kalina RE, Hodson WA: Transient cataracts in low birth weight infants. J Pediatr 82:314, 1973 206. Gruenwald P: Infants of low birth weight among 5,000 deliveries. Pediatrics 34:15, 1964 207. Mujsce DJ, Palmer C: Common neonatal illnesses. In HoekelmanRA, editor: Primary Pediatric Care, p. 597. Philadelphia, Mosby, 2001 208. AmericanAcademy of Pediatrics, Prevention of neonatal opthalmia. In Pickering LK (ed): 2000 Red Book: Report of the Committee on Infectious Diseases, 25th ed, p. 741. Elk Grove Village, IL, American Academy of Pediatrics, 2000 209. Crede R: Reports from the obstetrical clinic in Leipzig. Prevention of eye inflammation
in the newborn. Am J Dis Child 121:3, 1971 210. Nishida H, Risemberg HM: Silver nitrate opthalmic solution and chemical conjunctivitis. Pediatrics 56:368, 1975 211. Isenberg SJ, Apt L, Wood M: A controlled trial of povidone-iodine as prophylaxis against opthalmia
neonatorum. N Engl J Med 332:562, 1995 212. Alexander ER, Harrison HR: Role of Chlamydia trachomatis in perinatal infection. Rev Infect Dis 5:713, 1983 213. Weiss SG, Newcomb RW, Beem MO: Pulmonary assessment of children after chlamydial pneumonia of infancy. J Pediatr 108:659, 1986 214. Hammerschlag MR: Chlamydial infections. J Pediatr 114:727, 1989 215. Rosales T, Leake R, Isenberg S, et al: Systematic effects of mydriatics in lower-weight infants. J Pediatr Opthalmol Strabismus 18:42, 1981 216. Isenberg S, Everett S: Cardiovascular effects of myodriatics in low-birth-weight
infants. J Pediatr 105:111, 1984 217. Mirmanesh SJ, Abbasi S, Bhutani VK: Alpha-adrenergic bronchoprovocation in neonates with bronchopulmonary
dysplasi. J Pediatr 121:622, 1992 218. Isenberg SJ, Abrams C, Hymen PE: Effects of cyclopentliate eyedrops on gastric secretory function in pre-term
infants. Opthalmology 92:698, 1985 219. Wallace DK, Steinkuller PG: Ocular medications in children. Clin Pediatr 37:645, 1998 220. Oski FA: Vitamin E: A radical defense. N Engl J Med 303:454, 1980 221. Bieri JG, Corash L, Hubbard VS: Medical uses of vitamin E. N Engl J Med 308:1063, 1983 222. Lemons JA, Maisels MJ: Vitamin E: How much is too much?Pediatrics 76:625, 1985 223. Zipursky A, Brown EJ, Watts J, et al: Oral vitamin E supplementation for the prevention of anemia in premature
infants: A controlled trial. Pediatrics 79:61, 1987 224. Kushner BJ, Essner D, CohenIL , et al: Retrolental fibroplasia. Part II. Pathologic correlation. Arch Ophthalmol 95:29, 1977 225. Ashton N: Oxygen and the retinal blood vessels. Trans Ophthal Soc UK 100:359, 1980 226. Hittner HM, Rudolph AJ, Kretzer FL: Suppression of severe retinopathy of prematurity with vitamin E supplementation: Ultrastructural
mechanism of clinical efficacy. Ophthalmology 91:1512, 1984 227. Johnson L: Retrolental fibroplasia. A new look at an unsolved problem. Hosp Pract 16:109, 1981 228. Johnson L, Quinn GE, Abbas S, et al: Effect of sustained pharmacologic vitamin E levels on incidence and severity
of retinopathy of prematurity: A controlled clinical trial. J Pediatr 114:827, 1989 229. Johnson L, Quinn GE, Abbasi S, et al: Severe retinopathy of prematurity in infants with birth weights less than 1250 grams: Incidence
and outcome of treatment with pharmacologic serum
levels of vitamin E in addition to cryotherapy from 1985 to 1991. J Pediatr 127:632, 1995 230. Raju TN, Langenberg P, Bhutani V, et al: Vitamin E prophylaxis to reduce retinopathy of prematurity: a reappraisal
of published trails. J Pediatr 131:844, 1997 231. Law MR, Wijewardene K, Wald NJ: Is routine vitamin E administration justified in very low-birthweight
infants. Dev Med Child Neurol 32:442, 1990 232. Schaffer DB, Johnson L, Quinn GE, et al: Vitamin E and retinopathy of prematurity: Follow-up at one year. Ophthalmology 92:1005, 1985 233. Wender DF, Thulin GE, Wolken Smith GJ, et al: Vitamin E affects lung biochemical and morphologic response to hyperoxia
in the newborn rabbit. Pediatr Res 15:262, 1981 234. Saldanha RL, Cepeda EE, Poland RL: The effect of vitamin E prophylaxis on the incidence and severity of bronchopulmonary
dysplasia. J Pediatr 101:89, 1982 235. Arrowsmith JB, Faich GA, Tomita DK, et al: Morbidity and mortality among low birth weight infants exposed to an intravenous
vitamin E product, E-ferol. Pediatrics 83:244, 1989 236. Neal PR, Erickson P, Baenziger JC, et al: Serum vitamin E levels in the very low birth weight infant during oral
supplementation. Pediatrics 77:636, 1986 237. Ehrenkranz RA: Vitamin E and retinopathy of prematurity: Still controversial. J Pediatr 114:801, 1989 238. Cremer RJ, Perryman PW, Richards DH: Influence of light on the hyperbilirubinemia of infants. Lancet 1:1094, 1958 239. Brown AK, Kim MH, Wu PYK, et al: Efficacy of phototherapy in prevention and management of neonatal hyperbilirubinemia. Pediatrics 75:393, 1985 240. Ennever JF, Knox I, Denne SC, et al: Phototherapy for neonatal jaundice. In vivo clearance of bilirubin photoproducts. Pediatr Res 19:205, 1985 241. Landry RJ, Schedt PC, Hammond RW: Ambient light and phototherapy conditions of eight neonatal care units: A
summary report. Pediatrics 75:393, 1985 242. Rubaltelli FF, Zanardo V,0Granati B: Effect of various phototherapy regimens on bilirubin decrement. Pediatrics 61:838, 1978 243. Tan KL: The pattern of bilirubin response to phototherapy for neonatal hyperbilirubinemia. Pediatr Res 16:670, 1982 244. Dobson V: Phototherapy retinal damage. Invest Ophthalmol 15:595, 1976 245. Messner KH, Maisels MJ, Loure-DuPree AE: Phototoxicity to the newborn primate retina. Invest Ophthalmol Vis Sci 17:1178, 1978 246. Messner KH: Light toxicity to newborn retina. Pediatr Res I2:530, 1978 247. Sykes SM, Robison WG, Waxier M, et al: Damage to the monkey retina by broad-spectrum fluorescent light. Invest Ophthalmol Vis Sci 20:425, 1981 248. Kalina RE, Forrest GL: Ocular hazards of phototherapy for hyperbilirubinemia. J Pediatr Ophthalmol 8:116, 1971 249. Dobson V, Cowett RM, Riggs LA: Long-term effects of phototherapy on visual function. J Pediatr 86:555, 1975 250. Bhupathy K, Sethupathy R, Pildes RS, et al: Electroretinography in neonates treated with phototherapy. Pediatrics 61:189, 1978 251. Dobson V, Riggs L, Siqueland ER: Electroretinographic determination of dark adaptation functions of children
exposed to phototherapy as infants. J Pediatr 85:25, 1974 252. Sherman SM: Development of interocular alignment in cats. Brain Res 37:187, 1972 253. Cyander M, Berman N, Hein A: Recovery of function in cat visual cortex following prolonged deprivation. Exp Brain Res 25:139, 1976 254. Cyander M: Interocular alignment following visual deprivation in the cat. Invest Ophthalmol Vis Sci 18:726, 1979 255. Weisel TN, Hubel OH: Comparison of the effects of unilateral and bilateral eye closure on cortical
unit responses in kittens. J Neurophysiol 28:1029, 1965 256. Hubel DH: The visual cortex of normal and deprived monkeys. Am Sci 67:532, 1979 257. Drew JH: Phototherapy: Short- and long-term complications. Arch Dis Child 51:454, 1976 258. Hoyt CS: The long-term visual effects of short-term binocular occlusion
of at-risk neonates. Arch Ophthalmol 98:1967, 1980 259. Hubel DH, Wiesel TN: The period of susceptibility to the physiological effects of unilateral
eye closure in kittens. J Physiol 206:419, 1970 260. von Noorden GK: New clinical aspects of stimulus deprivation amblyopia. Am J Ophthalmol 92:416, 1981 261. Larsen JS: The sagittal growth of the eye. Part IV. Ultrasonic measurement of the
axial length of the eye from birth to puberty. Acta Ophthalmol 49:873, 1971 262. Gordon RA, Donzis P: Refractive development of the human eye. Arch Ophthalmol 103:785, 1985 263. Swan K, Wilkins JH: Extraocular muscle surgery in early infancy: Anatomical factors. J Pediatr Ophthalmol Strabismus 21:44, 1984 264. Blomdahl S: Ultrasonic measurements of the eye in the newborn infant. Acta Ophthalmol 57:1048, 1979 265. Sorsby A, Sheridan M: The eye at birth: Measurement of the principal diameters in forty-eight
cadavers. J Anat 94:192, 1960 266. Inagaki Y: The rapid change of corneal curvature in the neonatal period and infancy. Arch Ophthalmol 104:1026, 1986 267. Donzis PB, Insler MS, Gordon RA: Corneal curvature in premature infants. Am J Ophthalmol 99:213, 1985 268. Horsten GPM, Winkelman JE: Electrical activity of the retina in relation to histological differentiation
in infants born prematurely and at full term. Vision Res 2:269, 1962 269. Mann I: The Development of the Human Eye, p. 114. London, Cambridge University Press, 1928 270. Isenberg SJ: Macular development in the premature infant. Am J Ophthalmol 101:74, 1986 271. Hendrickson AE, Yuodelis C: The morphological development of the human fovea. Ophthalmology 91:603, 1984 272. Hickey TL: Postnatal development of the human lateral geniculate nucleus: Relationship
to a critical period for the visual system. Science 198:836, 1977 273. Magoon EH, Robb RM: Development of myelin in human optic nerve and tract. Invest Ophthalmol Vis Sci 19:3, 1980 274. Conel J: The Postnatal Development of Human Cerebral Cortex, Vol. I, The Cortex of the Newborn. Cambridge, Harvard University Press, 1939 275. Ingram RM: Refraction of 1-year-old children after atropine cycloplegia. Br J Ophthalmol 63:343, 1979 276. Banks MS: Infant refraction and accommodation. Int Ophthalmol Clin 20:205, 1980 277. Glickstein M, Millodot M: Retinoscopy and eye size. Science 168:605, 1970 278. Harter MR, Deaton FK, Odom JV: Maturation of evoked potentials and visual preference in 6–45 day
old infants: Effects of check size, visual acuity and refractive error. Electroencephalogr Clin Neurophysiol 42:595, 1977 279. Scharf J, Zonis S, Zeltzer M: Refraction in Israeli premature babies. J Pediatr Ophthalmol 12:193, 1975 280. Scharf J, Zonis S, Zeltzer M: Refraction in premature babies: A prospective study. J Pediatr Ophthalmol 15:48, 1978 281. Fulton AB, Dobson V, Salem D, et al: Cycloplegic refractions in infants and young children. Am J Ophthalmol 90:239, 1980 282. Sorsby A, Sheridan M, Leary GA, et al: Vision, visual acuity, and ocular refraction of young men: Findings in
a sample of 1,033 subjects. BMJ 1:1394, 1960 283. Mohindra I, Held R, Gwiazda J, et al: Astigmatism in infants. Science 202:329, 1980 284. Howland HC, Sayles N: Photorefractive measurements of astigmatism in infants and young children. Invest Ophthalmol Vis Sci 25:93, 1984 285. Hsu-Winges C, Hamer RD, Norcia AM, et al: Polaroid photorefractive screening of infants. J Pediatr Ophthalmol Strabismus 26:254, 1989 286. Mitchell DE, Freeman RD, Millodot M, et al: Meridional amblyopia: Evidence for modification of the human visual system
by early experience. Vision Res 13:535, 1973 287. Dobson V, Fulton AB, Manning K, et al: Cycloplegic refractions of premature infants. Am J Ophthalmol 91:490, 1981 288. Shapiro A, Yanko L, Nawratzki I, et al: Refractive power of premature children at infancy and early childhood. Am J Ophthalmol 90:234, 1980 289. Nissenkorn I, Yassur Y, Mashkowski D, et al: Myopia in premature babies with and without retinopathy of prematurity. Br J Ophthalmol 67:170, 1983 290. Gwiazda J, Scheiman M, Mohindra I, et al: Astigmatism in children: Changes in axis and amount from birth to six years. Invest Ophthalmol Vis Sci 25:88, 1984 291. Rutstein RP, Wesson MD, Gotlieb S, et al: Clinical comparison of the visual parameters in infants with intrauterine
growth retardation vs. infants with normal birth weight. Am J Optom Physiol Opt 63:697, 1986 292. Banks MS: The development of visual accommodation during early infancy. Child Dev 51:646, 1980 293. Braddick O, Atkinson J, French J, et al: A photorefractive study of infant accommodation. Vision Res 19:1319, 1979 294. Haynes HM, White BL, Held R: Visual accommodation in human infants. Science 148:528, 1965 295. Powers MK, Dobson V: Effect of focus on visual acuity of human infants. Vision Res 22:521, 1982 296. Atkinson J, Braddick O, Moar K: Development of contrast sensitivity over the first three months of life
in the human infant. Vision Res 17:1037, 1977 297. Salapetek P, Bechtold AG, Bushnell EW: Infant visual acuity as a function of viewing distance. Child Dev 47: 860, 1976 298. Aslin RN, Jackson RW: Accommodative-convergence in young infants: Development of a synergistic
sensory motor system. Can J Psychol 33:222, 1979 299. Hoyt CS, Nickel BL, Billston FA: Ophthalmological examination of the infant: Developmental aspects. Surv Ophthalmol 26:177, 1982 300. Isenberg SJ, Dang Y, Jotlerand V: The pupils of term and preterm infants. Am J Ophthalmol 108:75, 1989 301. Isenberg SJ, Molarte A, Vazquez M: The fixed and dilated pupils of premature neonates. Am J Ophthalmol 110:168, 1990 302. Frank JW, Kushner BJ, France T: Paradoxic pupillary phenomena: A review of patients with pupillary constriction
to darkness. Arch Ophthalmol 106:1564, 1988 303. Robinson RJ: Assessment of gestational age by neurological examination. Arch Dis Child 41:437, 1966 304. Nelson LB, Rubin SE, Wagner RS, et al: Developmental aspects in the assessment of visual function in young children. Pediatrics 73:375, 1984 305. Goren CC, Sarty M, Wu PYK: Visual following and pattern discrimination of facelike stimuli by newborn
infants. Pediatrics 56:544, 1975 306. Friendly DS: Visual acuity assessment of the preverbal patient. In Isenberg SJ (ed): The Eye in Infancy, pp. 48–56. Chicago, Year Book Medical Publishers, 1989 307. Aslin RN, Salapatek P: Saccadic localization of peripheral targets by the very young human infant. Percept Psychophys 17:293, 1975 308. Eviatar L, Miranda S, Eviatar A, et al: Development of nystagmus in response to vestibular stimulation in infants. Ann Neurol 5:508, 1979 309. Hoyt CS, Jastrzebski G, Marg E: Delayed visual maturation in infancy. Br J Ophthalmol 67:127, 1983 310. Mellor DH, Fielder AR: Dissociated visual development: Electrodiagnostic studies in infants who
are “slow to see.”Dev Med Child Neurol 22:327, 1980 311. Kivlin JD, Bodnar A, Ralston CW, et al: The visually inattentive preterm infant. J Pediatr Ophthalmol Strabismus 27:190, 1990 312. Linksz A: Visual acuity in the newborn with notes on some objective methods to determine
visual acuity. Doc Ophthalmol 34:259, 1973 313. Dobson V, Teller DY: Visual acuity in human infants: Physiological studies. Vision Res 18:1469, 1978 314. Fulton AB, Hansen RM, Manning KA: Measuring visual acuity in infants. Surv Ophthalmol 25:325, 1981 315. Dobson V: Behavioral tests of visual acuity in infants. Int Ophthalmol Clin 20:233, 1980 316. Sokol S: Pattern visual evoked potentials: Their use in pediatric ophthalmology. Int Ophthalmol Clin 20:251, 1980 317. Teller DY, McDonald M, Preston K, et al: Assessment of visual acuity in infants and children: The acuity card procedure. Dev Med Child Neurol 28:779, 1986 318. Westall CA, Ainsworth JR, Buncic JR: Which ocular and neurologic conditions cause disparate results in visual
acuity scores recorded with visually evoked potential and teller acuity
cards?J AAPOS 4:295, 2000. 319. Norcia AM, Tyler CW: Spatial frequency sweep VEP: Visual acuity during the first year of life. Vision Res 25:1399, 1985 320. Harter MR, Suitt CD: Visually-evoked cortical responses and pattern vision in the infant: A
longitudinal study. Psychon Sci 18:235, 1970 321. Marg E, Freeman DN, Peltzman P, et al: Visual acuity development in human infants: Evoked potential measurements. Invest Ophthalmol 15:150, 1976 322. Sokol S, Dobson V: Pattern reversal visually evoked potentials. Invest Ophthalmol 15:58, 1976 323. Sokol S: Measurement of infant visual acuity from pattern reversal evoked potentials. Vision Res 18:33, 1978 324. Dayton GO, Jones MH, Aiu P, et al: Developmental study of coordinated eye movements in the human infant. Part
I. Visual acuity in the newborn human: A study based on induced optokinetic
nystagmus recorded by electro-oculography. Arch Ophthalmol 71:865, 1964 325. Gorman JJ, Cogan DO, Gellis SS: An apparatus for grading the visual acuity on the basis of optico-kinetic
nystagmus. Pediatrics 19:1088, 1957 326. Gorman JJ, Cogan DG, Gellis SS: A device for testing visual acuity in infants. Sight Sav Rev 29:80, 1959 327. Kiff RD, Lepard C: Visual responses of premature infants. Arch Ophthalmol 75:631, 1966 328. Frantz RL, Ordy JM, Udelf MS: Maturation of pattern vision in infants during the first six months. J Comp Physiol Psychol 55:907, 1962 329. Berlyne DE: The influence of the albedo and complexity of stimuli on visual fixation
in the human infant. Br J Psychol 49:315, 1958 330. Frantz RL: Pattern vision in young infants. Psychol Rec 8:43, 1958 331. Teller DY, Morse R, Borton R, et al: Visual acuity for vertical and diagonal gratings in human infants. Vision Res 14:1433, 1974 332. Mayer DL, Dobson V: Assessment of vision in young children: A new operant approach yields estimates
of acuity. Invest Ophthalmol Vis Sci 19:566, 1980 333. Frantz RL, Fagan JF, Miranda SB: Early visual selectivity as a function of pattern variables, previous exposure, age
from birth and conception, and expected cognitive deficit. In Cohen LB, Salapatek P (eds): Infant Perception: From Sensation To Cognition, Vol. I. Basic Visual Process, p. 249. New York, Academic Press, 1975 334. Mayer DL, Fulton AB, Rodier D: Grating and recognition acuities of pediatric patients. Ophthalmology 91:947, 1984 335. Friendly DS, Jaafar MS, Morillo DL: A comparative study of grating and recognition visual acuity testing in
children with anisometropic amblyopia without strabismus. Am J Ophthalmol 110:293, 1990 336. Frantz RL, Miranda SB: Newborn infant attention to form of contour. Child Dev 46:224, 1975 337. Slater A, Sykes M: Newborn infants' visual responses to square wave gratings. Child Dev 48:545, 1977 338. Grelotti DJ, Gauthier I, Schultz RT: Social interest and the development of cortical face specialization: What
autism teaches us about face processing. Dev Psychobiol 40:213, 2002 339. Ballantyne AO, Trauner DA: Facial recognition in children after perinatal stroke. Neuropsychiatry Neuropsychol Behav Neurol 12:82, 1999 340. Nachson I: On the modularity of face recognition: The riddle of domain specificity. J Clin Exp Neuropsychol 17:256, 1995 341. Milewski AE: Infants' discrimination of internal and external pattern elements. J Exp Child Psychol 22:229, 1976 342. Maurer D, Lewis TL: A physiological explanation of infants' visual development. Can J Psychol 33:232, 1979 343. Bower TGR: The object in the world of the infant. Sci Am 225:30, 1971 344. Dodwell PC, Muri DW, DiFranco D: Infant perception of visually presented objects. Science 203:1138, 1979 345. Dodwell PC, Muir DW, DiFranco D: Responses of infants to visually presented objects. Science 194:209, 1976 346. Bower TGR, Dunkeld J, Wishart JG: Infant perception of visually presented objects. Science 203:1137, 1979 347. Aslin RN, Dumais ST: Binocular vision in infants: A review and theoretical framework. Adv Child Dev Behav 15:54, 1980 348. Aslin RN: Development of binocular fixation in human infants. J Exp Child Psychol 23:133, 1977 349. Fox R, Aslin RN, Shea SL, et al: Stereopsis in human infants. Science 207:323, 1980 350. Archer SM, Helveston EM, Miller KK, et al: Stereopsis in normal infants and infants with congenital esotropia. Am J Ophthalmol 101:591, 1986 |