EXTERNAL ADNEXAL TISSUES
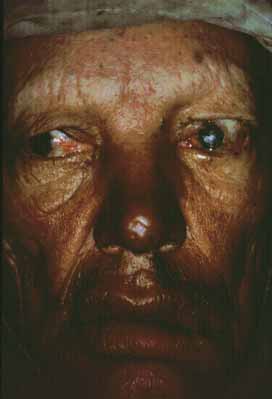
Brow loss is one of the most common manifestations of leprosy41–43 and occurs more frequently in the tuberculoid form (Fig. 1). Brow loss begins temporally and progresses nasally, perhaps because the temporal brow is somewhat cooler. Madarosis, or eyelash loss, is also common, affecting the lower lid before the upper lid. The severity of both brow loss and madarosis is related to the duration of disease.44
Seventh cranial nerve paralysis is common and results in lagophthalmos and ectropion of the lower eyelid (see Fig. 1). When fifth-nerve involvement also occurs, a marked form of anesthetic corneal exposure may result. It has been believed that inflammation caused by leprosy may cause upper lid entropion,43,45 but trachoma is often endemic in these populations so the upper lid deformity may often be caused by trachoma. Unfortunately, multibacillary patients develop paresis later in their disease when they often have corneal sensory loss. These patients have little urge to blink, nor can they effectively blink when they try. Such patients are at high risk for corneal ulceration, and surgical intervention should be considered early.
Eyelid nodules, ENL, and erysipeloid reactions of the lid are recognized less frequently, probably because they occur less frequently, or perhaps because they are transient. Loss of skin elasticity, infiltration of the marginal and pretarsal fibers of the orbicularis oculi muscle by M. leprae, and loss of muscle tone contributes to dermatochalasis and heavy, drooping upper lids (see Fig. 1). Further atrophy at the canthal tendons and tarsal plates create heavy, floppy lids allowing ectropion or perhaps entropion, and trichiasis.
Meibomian gland infiltration leads to atrophy and an inadequate lipid production with associated tear dysfunction. Leprosy involves the lacrimal gland by causing denervation of the lacrimal gland that helps to contribute to a dry-eye syndrome. Leprotic dacryocystitis secondary to nasal disease is a common complication, and may contribute to subsequent corneal ulceration and microbial keratitis.
CORNEAL AND CONJUNCTIVAL INVOLVEMENT
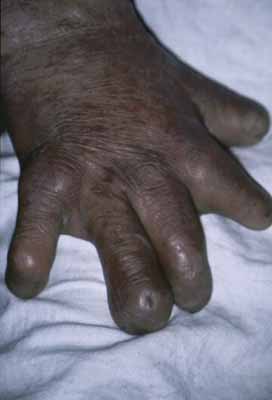
Exposure keratitis occurs frequently in patients with leprosy. Seventh cranial nerve involvement with lagophthalmos results in exposure keratitis inferiorly. Fifth cranial nerve involvement with an anesthetic cornea and decreased blinking also contributes to the problem. Patients may inadvertently traumatize themselves by rubbing their eyes with calloused, insensitive, and often infected fingers (Fig. 2). This constant exposure is often combined with lid deformities (trichiasis) so that corneal ulceration and secondary bacterial infection are frequent events.46 These ulcers may perforate, leading to phthisis and blindness. Even when the corneal ulcer heals, there is stromal scarring and thinning. Corneal hyposensitivity or anesthesia is seen more commonly in lepromatous leprosy, and is coincident with more severe complications.47
|
Enlarged, edematous corneal nerves are found in lepromatous leprosy. The nerve involvement has the appearance of focal swelling resembling beads on a string and consists of masses of M. leprae and a surrounding granulomatous response within the corneal nerve. These nerve swellings are pathognomonic of leprosy and may be the first ocular or systemic finding.48
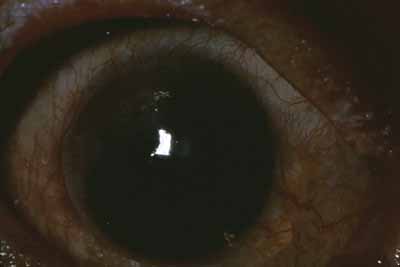
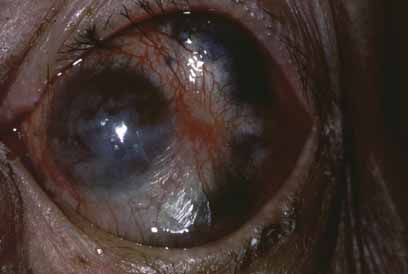
There is also avascular or punctate keratitis consisting of chalky, white, punctate subepithelial opacities that first form in the superior temporal quadrant of the cornea. These are miliary lepromas with macrophages, lymphocytes, and M. leprae.49 The individual lesions gradually become less demarcated with a surrounding haze and eventually becoming confluent. Histologically within these nebulae, discrete calciumlike deposits may be seen, often associated with destruction of Bowman's layer. Superficial neovascularization occurs late in the disease to produce the classic leprous pannus (Fig. 3).49 The bacilli, which produces avascular keratitis, probably reach the cornea by way of the nerves, but deposits also occur adjacent to trachomatous pannus vessels, so that a vascular route is also possible.
|
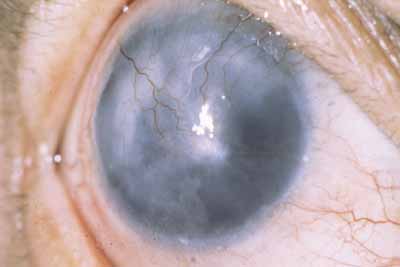
Corneal lepromas (Fig. 4) occur more commonly in South America50 and Japan51 but are relatively infrequent. These lesions are large granulomas with epithelioid cells, lymphocytes, and bacteria. They may become large enough to involve the visual axis and compromise vision. Lepromas usually arise at the limbus (most commonly the lateral limbus) or on the sclera and then encroach onto the cornea.
Interstitial keratitis begins in the superior temporal quadrant or in the superior quadrants. It is probably a more severe form of the avascular keratitis, with necrosis and then vascular invasion. Interstitial keratitis may begin without a preceding avascular keratitis in the midstroma of the cornea (superior nasal quadrant) or as a discoid lesion often located superiorly. The interstitial keratitis may progress to involve the visual axis (Fig. 5), with a decrease in visual acuity. Ghost vessels are occasionally seen in mid- to deep stroma in interstitial keratitis, indicating previous inflammatory activity.
SCLERAL AND UVEAL LESIONS
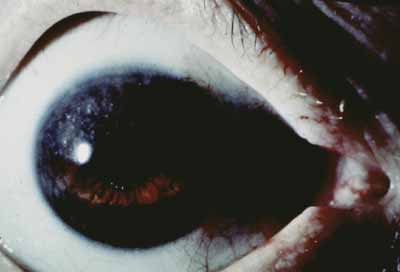
Episcleritis, scleritis, and uveitis are the initial presenting complaints in up to 16% of leprosy patients.52 The nodular episcleritis that occurs consists of focal leproma with surrounding inflammatory response. There are also more diffuse forms of episcleritis. Episcleritis commonly occurs as part of the ENL reaction, and may be associated with iridocyclitis. Lepromas may occur in the interpalpebral fissure more commonly because this area is cooler. The episcleritis and scleritis may be the result of direct bacillary invasion, but there is strong evidence that some cases may be mediated by immune responses, such as the deposition of immune complexes. Chronic or recurrent scleritis in leprosy may result in scleromalacia and staphyloma (Fig. 6) and, in some cases, disorganization of the globe.
Uveitis in leprosy may be the result of several different mechanisms. Uveitis with the erythema nodosum leprosum reaction probably results from a hypersensitivity reaction (probably antigen-antibody mediated)25 and usually occurs with therapy or occasionally after termination of therapy. This iridocyclitis, which also occurs spontaneously, has an abrupt onset and fulminating course with hypopyon, posterior synechiae, and elevated intraocular pressure. Occasionally, there is a spontaneous hyphema presumably because of fragile vessels from previous attacks. This granulomatous uveitis is usually associated with greasy, keratic precipitates.
There is also a chronic, low-grade uveitis seen commonly in lepromatous leprosy, which is the principal cause of blindness in ocular leprosy.33,34 This insidious anterior uveitis causes gradual iris atrophy as manifested by loss of iris stroma with a moth-eaten appearance, frank iris holes (Fig. 7), and small, nonreactive pupils. In this condition, iris stromal nerve involvement and disintegration of iris stroma associated with leprosy bacilli can be demonstrated histologically.53 In this “white” iritis, patients have few anterior chamber cells and mild to moderate anterior chamber flare with minimal ciliary flush or conjunctival injection. There are a few scattered, fine, white keratic precipitates. Posterior synechiae are not common and, when present, may be the result of a previous attack of acute iritis. The dilator muscle is affected earlier and to a greater extent than the sphincter muscle, resulting in a small, nonreactive pupil.54 The pinpoint pupil may lead to marked visual loss if there is a corneal or lens opacity. This chronic, low-grade uveitis is more common in patients with lepromatous leprosy, although it may be present in those with tuberculoid leprosy.35
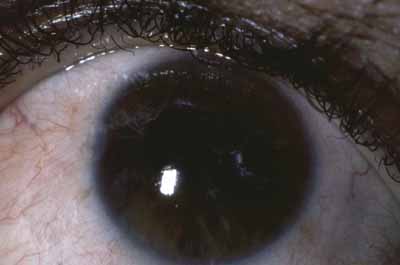
Iris pearls arise from the surface connective tissue of the iris and can be recognized on the iris surface even early in the disease (Fig. 8). These creamy white particles consist of bacilli and monocytes and are rarely associated with an inflammatory response.49 Iris pearls are pathognomonic of ocular leprosy and look like grains of sand on the iris surface, often occurring near the pupillary border. After several years, they may coalesce and drop into the inferior angle, where they can be observed by gonioscopy. Pearls have been reported on the retina55 but probably represent the migration of such spheres to the posterior segment rather than formation there.
|
The ciliary body may represent the site of bacillary activity in early leprosy. Several clinical signs suggest ciliary body dysfunction early in the disease. Premature presbyopia in lepromatous leprosy is common and is probably caused by a parasympathetic neuropathy affecting accommodation.56,57 Low intraocular pressures has been observed in some studies with a large fluctuation in postural intraocular pressure change early in the disease without other evidence of ocular involvement.58–60 Decreased intraocular pressure can also be found in household contacts of leprosy patients compared to endemic controls.61
Rarely, there may be a uveal effusion associated with an overlying scleritis and episcleritis. Choroiditis has been reported,62,63 but it is doubtful if leprosy was the cause.
Glaucoma is a result of seclusion of the pupil by posterior synechiae and resulting iris bombé. This condition is readily corrected by peripheral iridectomy or pupil transfixion. There is also a higher rate of elevated intraocular pressure in leprosy patients with a deep anterior chamber. It is not yet known if this is caused by peripheral anterior synechiae or by other forms of involvement of the outflow channels.