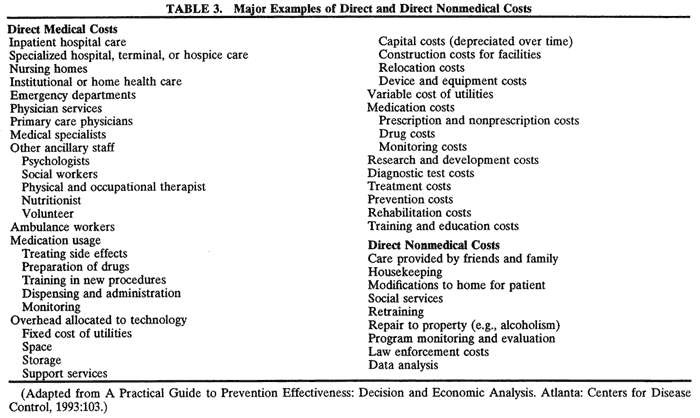
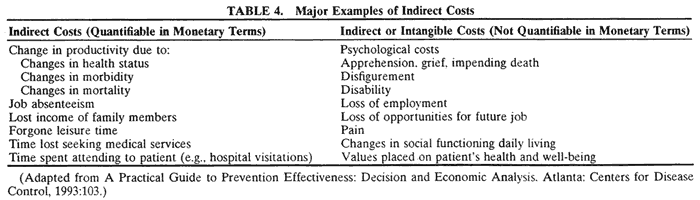
There are three main principles of efficiency:
- Do not waste scarce resources.
- Produce each output at the lowest possible cost.
- Produce those goods and services that people value the most.
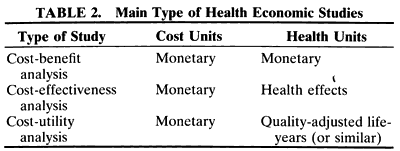
When some portion of those resources can be used to produce other goods and services, then the system is deemed to be operating at “technical efficiency.” When one has to increase the resources, one has to commit to a given venture, then one is said to be technically inefficient. “Allocative efficiency,” by contrast involves taking into account the concept of cost-effectiveness (CE), in which the decision taken is essentially an economically sound one. As such, allocative efficiency hinges on making appropriate value judgments about how to allocate resources. To assist us, the famous late-nineteenth century economist, Vilfredo Pareto, proposed that allocative efficiency is “attained when it is not possible to change the allocation of resources to make any one person better off without making at least one other person worse off.” Furthermore, one can identify two factors that modify or influence efficiency, namely (1) individual welfare, in which the individual decides which goods and services to consume, and (2) the available level and distribution of income in the economy. Hence, the Pareto criterion may be restated to read “that there is no unique allocation of resources such that there is only one allocatively efficient solution. Instead, there are a range of efficient allocations, one for each different combination of wealth and income.” Overall, the concept of the Pareto criterion is more theoretical than practical, since most changes in resource allocation have negative consequences and do, in fact, make certain segments of society worse off; that is, for any health policy change, there are both winners and losers.
Essentially, health economics is concerned with discovering the best or most efficient means of allocating scarce healthcare resources to yield a given healthcare policy objective, such as reducing infant mortality, and so forth. The concept of equity or fairness significantly affects the decision to pursue various healthcare strategies or interventions and often is at odds with the concept of allocative efficiency. At this point, it is worth introducing the concept of “distributive equity,” since it forces decision makers to be concerned with where the final healthcare resources end up rather than how the resources are apportioned. In this respect, it is further useful to consider the twin concepts of horizontal and vertical equity in our dissection of the concept of distributive equity.
Horizontal equity relates to the manner in which the scholars of the day believed that it was important to distribute an equal amount of goods and services to individuals who have similar economic circumstances. Under this view, the aim of efficiency is to achieve an equal distribution of healthcare resources to ensure that individuals with similar healthcare needs and costs obtain a similar amount on a per-capita basis. Vertical equity, by contrast, relates to the desire to distribute unequal amounts of healthcare resources among differently situated individuals in relation to the degree to which they are experiencing different healthcare situations. Thus, under this view, those segments of society with greater healthcare needs would receive a greater share of the total healthcare budget. The goal of health economics is to attempt to quantify the potential end users of such healthcare resources and so determine what sort of strategy is to be pursued, be it striving toward either horizontal or vertical equity.
Traditionally, health economists have been guided in their attempts to allocate scarce resources efficiently to maximize an individual's human capital. However, the human capital approach, as it is known, is not without its detractors, since it fails to take into account a number of important factors. Sick and mentally or physically disabled persons are always undervalued in terms of economic productivity to the economy. In addition, the human capital approach fails to take into account such factors as the wage gap, especially between the genders and different ethnic groups. As a consequence, some economists have argued that days of healthy life gained through the introduction of a particular healthcare intervention or technique need to be weighted according to the, age, sex, and ethnic group wage rate for those persons who are affected.1
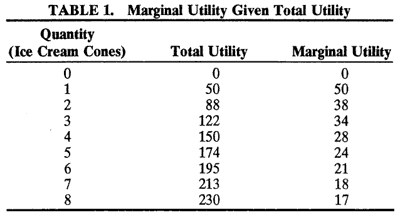
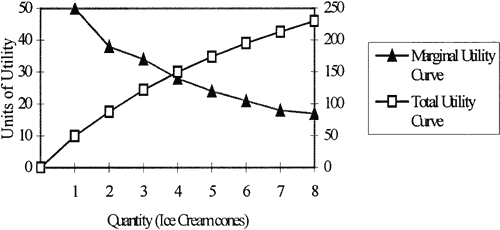
An equally fundamental principle of economics is the concept of “utility maximization,” in which each individual consumer seeks to derive as much satisfaction or happiness from the consumption of goods and services as possible. However, eventually, each consumer reaches a point at which he or she no longer derives the same degree of utility or satisfaction from the consumption of a given bundle of goods and services. The point beyond which individuals cease to derive increasing utility is called the law of diminishing marginal utility (Table 1 and Fig. 1). For example, consider the case of little Johnny eating an ice cream cone. After the first or second cone, Johnny's utility probably is still quite high; however, by the ninth or tenth ice cream cone, Johnny probably is feeling quite sick. Alas the law of diminishing marginal utility has a real-life example.