| Patients with dysthyroid orbitopathy may require orbital decompression
for a variety of reasons. Optic neuropathy in DO can cause severe visual
loss that is usually due to compression of the optic nerve from swelling
of the rectus muscles near the apex of the orbit.10 With adequate orbital decompression, this can frequently be reversed. Exposure
keratopathy from extreme proptosis can be quite severe, occasionally
leading to corneal perforation. Again, orbital decompression can
reduce the severity of exposure and therefore benefit patients with
extreme proptosis. Orbital decompression may also be performed for cosmesis, particularly
in those situations in which there is marked asymmetry
in the exophthalmos. The most common approach for orbital decompression for DO is to remove
portions of one or more of the bony walls of the orbit. This accomplishes
decompression by expanding the volume available to the orbital fat
and extraocular muscles. A variety of procedures have been described, including
removal of the lateral wall, the orbital floor, the medial
wall, and portions of the roof.11–16 Most surgeons use a variety of these techniques, alone or in combination, depending
on the patient's findings, including the amount of
proptosis, the presence of optic neuropathy, and pre-existing strabismus.17 Orbital fat excision without bone removal has been advocated for patients
with symptoms related to proptosis but without compressive optic neuropathy.18 The surgical approach should be selected after consideration of the patient's
findings, including the amount of proptosis, pre-existing
strabismus, and presence or absence of optic neuropathy. Lateral orbital
wall removal alone usually will produce a reduction in proptosis of 1 to 3 mm
and is less often associated with postoperative strabismus, but
it does not usually relieve the apical crowding causing optic neuropathy. Removal
of the medial wall is more effective in treating optic
neuropathy and produces an enophthalmic effect of 2 to 4 mm, but it
is more often accompanied by postoperative strabismus. Removal of the
floor also produces a larger enophthalmic effect of 3 to 5 mm; this procedure
is frequently accompanied by worsening of strabismus and/or numbness
or paresthesia in the distribution of the second division of the
fifth cranial nerve, the latter of which usually resolves after several
months. Combining a lateral wall removal with a medial wall and floor
procedure may enable the surgeon to improve the enophthalmic effect
to 6 to 10 mm without increasing the risk of induced strabismus. Removal
of portions of the orbital roof is reserved for those cases with
severe proptosis; it is possible to achieve a reduction in exophthalmos
of 10 to 17 mm with a panorbital decompression.16 SURGICAL TECHNIQUE General anesthesia is used for all orbital decompressions for dysthyroid
ophthalmopathy. If a four-wall decompression is being performed, we
recommend that a neurosurgeon familiar with cranio-orbital junction surgery
be a part of the operating team. Dexamethasone phosphate, 10 to 40 mg, is
given intravenously at the beginning of the surgery. We do not
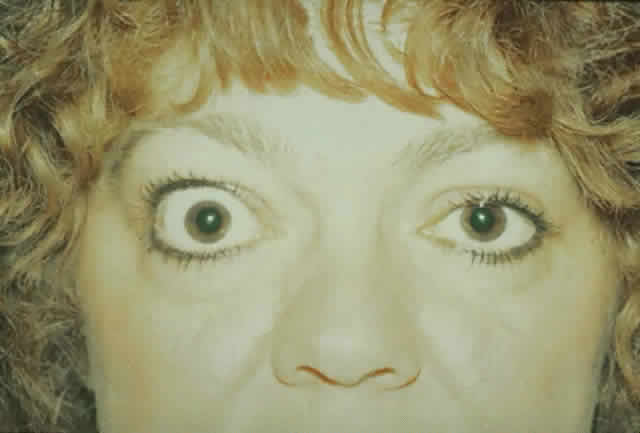
routinely use prophylactic antibiotics for orbital decompression. ONE- AND TWO-WALL DECOMPRESSION When the degree of proptosis is not severe and optic neuropathy is present, a
medial wall and sometimes a medial orbital floor decompression
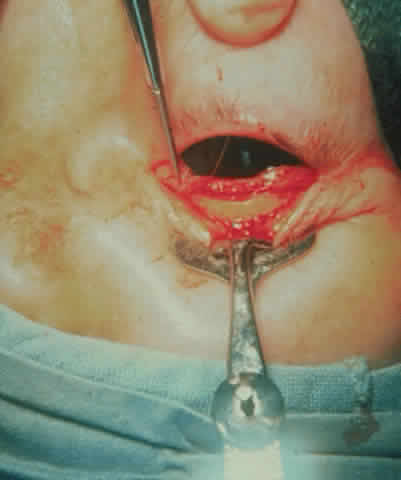
is indicated (Fig. 15). As indicated earlier, a Caldwel-Luc approach may be used to accomplish
this, but we prefer the medial approach because of a lesser incidence
of diplopia. This has been done by an endoscopic method, which may
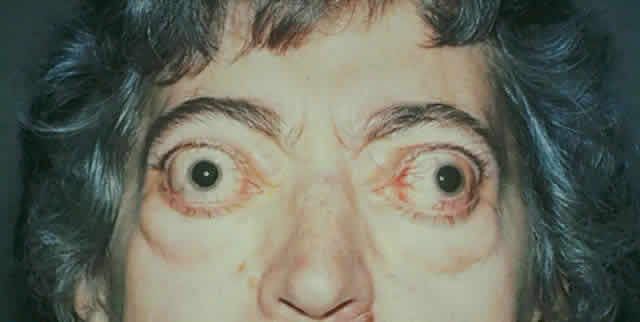
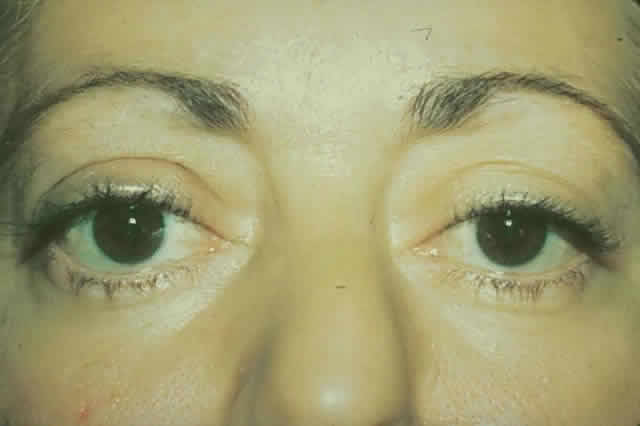
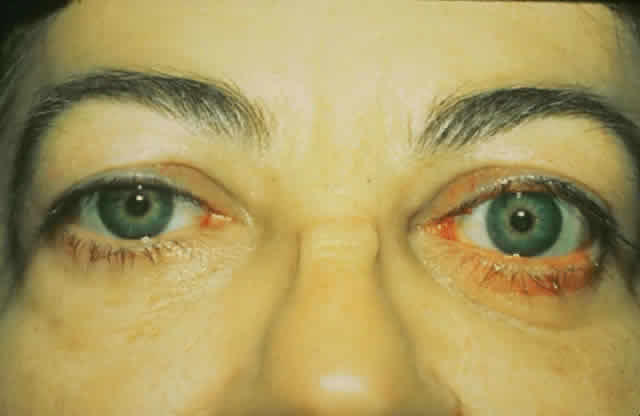
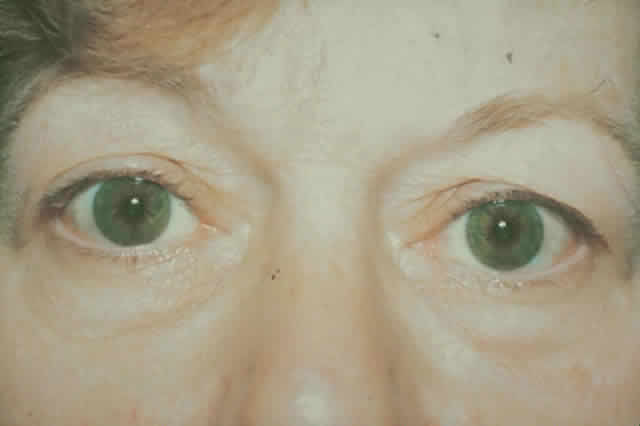
gain favor in the future. 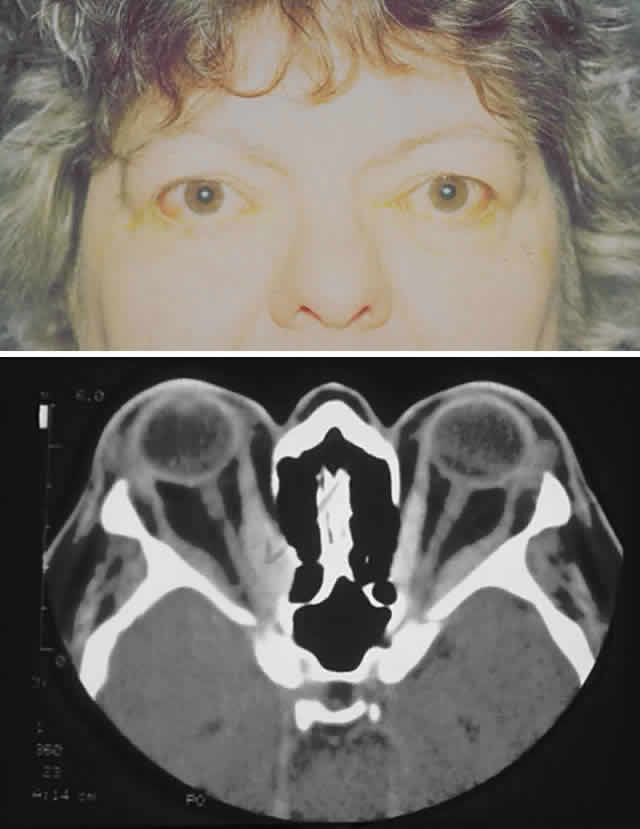 Fig. 15. A. A 48-year-old woman with dysthyroid optic neuropathy. Visual acuity
right eye 6/24, left eye 6/9. Color vision right eye 1/12, left eye 10/12--Ishihara
plates. Goldmann visual fields showed central scotomas in
both eyes. Hertel measurements right 25, left 24, base 102. B. Preoperative
axial CT scans showing apical crowding and proptosis. Fig. 15. A. A 48-year-old woman with dysthyroid optic neuropathy. Visual acuity
right eye 6/24, left eye 6/9. Color vision right eye 1/12, left eye 10/12--Ishihara
plates. Goldmann visual fields showed central scotomas in
both eyes. Hertel measurements right 25, left 24, base 102. B. Preoperative
axial CT scans showing apical crowding and proptosis.
|
A medial approach is made through an extended Lynch incision halfway between
the medial canthus and the nose, extending from the superior lacrimal
crest to 1 cm below the medial brow. The dissection through the
skin is done with a No. 15 Bard Parker knife, and the subcutaneous tissue
is dissected with cutting cautery down to the periosteum. The periosteum
is incised and reflected off the medial orbital wall with a periosteal
elevator. Then a Hall drill is used to make a small opening in
the anterior ethmoid sinus. A kerasin and a rongeur are then used to
remove the medial orbital wall and ablate the ethmoid air cells back to
the sphenoid sinus. Care is taken to stay below the anterior and posterior
ethmoidal arteries to prevent excessive bleeding and to avoid the
cribriform plate. CT scans are displayed in the operating room for
study of the anatomy of the cribriform plate prior to surgery. At times, the
plate is lower than usual and easily entered if not anticipated. If
additional decompression is desired, the medial orbital floor is
then removed with rongeurs to the inferior neurovascular bundle. Hemostasis
is achieved as well as possible and drainage is obtained through
the nostril on the side of the lesion. The deep tissues on the side of
the nose are closed with 4-0 Vicryl sutures in an interrupted manner. The
skin is closed with a running 6-0 plain catgut suture. A pressure
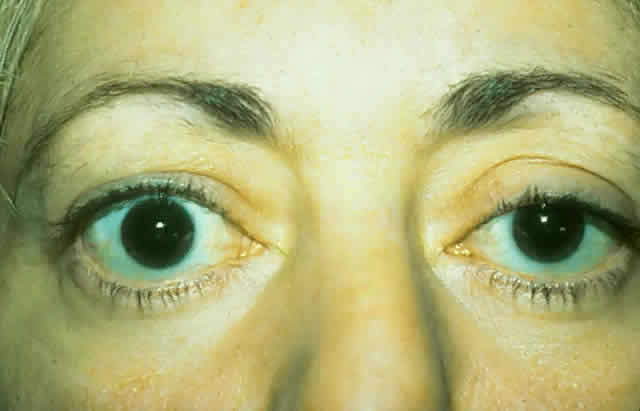
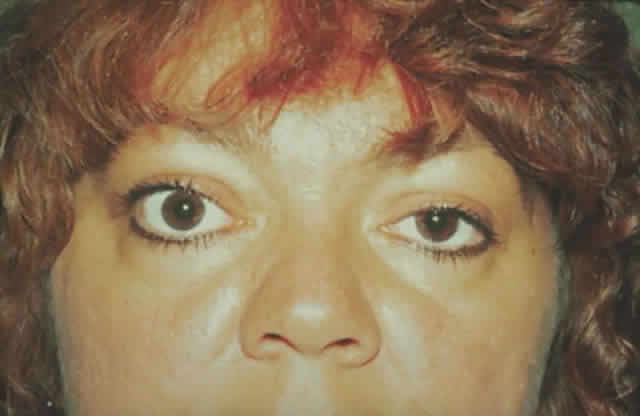
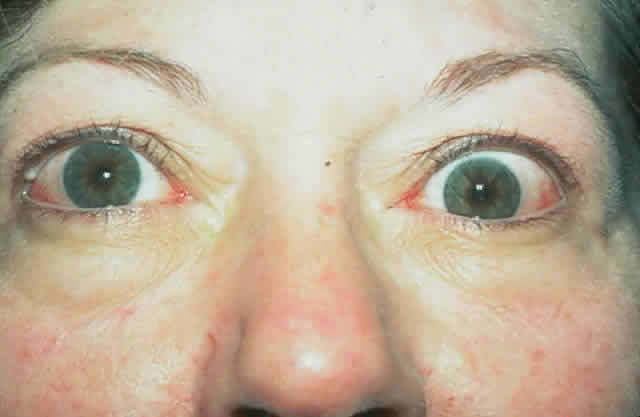
dressing is applied. The patient (see Fig. 15) had a 6 mm decompression (Fig. 16). 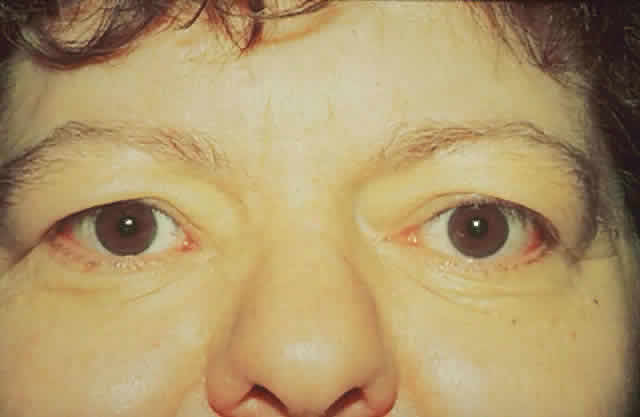 Fig. 16. Postoperative appearance of woman in Figure 15. Visual acuity right eye 6/9, left eye 6/6. Color vision right eye 7/12, left
eye 12/12--Ishihara plates. Goldmann visual field showed only
enlarged blind spot right eye. Hertel measurements right 19, left 18. Fig. 16. Postoperative appearance of woman in Figure 15. Visual acuity right eye 6/9, left eye 6/6. Color vision right eye 7/12, left
eye 12/12--Ishihara plates. Goldmann visual field showed only
enlarged blind spot right eye. Hertel measurements right 19, left 18.
|
THREE- AND FOUR-WALL DECOMPRESSION After administration of general anesthesia, with the patient in a supine
position, the head is turned approximately 45° to the contralateral
side. A 35-mm line is drawn from the lateral canthus posteriorly toward
the upper attachment of the ear to the head, and this area is injected
with 2% Xylocaine and 1:100,000 epinephrine. The skin is incised
along this line to the level of the temporalis fascia and then widely
undermined. Anteriorly, the periosteum of the lateral orbital rim is
incised 2 mm posterior to the rim for a length of 35 mm with relaxing
incisions at the superior and inferior extents. The periosteum is reflected
off the underlying bone along the rim, and the temporalis muscle
is reflected posteriorly by use of the cutting cautery. The periosteum
is elevated anteriorly to the rim and the periorbita is dissected free
from the underlying bone. This is firmly adherent only at the lateral
orbital tubercle where the lateral canthal structures attach. Cautery
may be used on the vascular structures exiting the orbit through the
zygomatic bone. With malleable retractors protecting the periorbita, the superior and inferior
lateral orbital rims are cut with a Stryker sagittal saw. The
rim is then grasped with a rongeur, outfractured, and removed. The rim
of the removed bone is thinned posteriorly with the high-speed air drill
and is set aside wrapped in saline-soaked gauze. Bone is removed back
to the greater wing of the sphenoid with use of rongeurs and a high-speed
air drill. At this point, if a four-wall decompression is being performed, bone removal
continues with the drill, exposing the temporal dura. Bone wax is
used to control bone bleeding. With malleable ribbon retractors protecting
the periorbita, the diamond-tipped drill removes bone superiorly
to expose dura in the roof of the orbit. The dura is dissected from
the orbital roof and protected with cottonoids. Small rongeurs are then
used to remove bone from the roof anteriorly to the orbital rim, laterally
and medially to the inner one third of the roof, avoiding the frontal
sinus, and posteriorly to the lesser wing of the sphenoid. The floor of the orbit is then exposed through a canthotomy and fornix
incision as follows: The inferior crus of the lateral canthal tendon is
severed and the conjunctiva incised with scissors in the fornix along
nearly its entire length. This incision is carried to the periosteum
of the inferior orbital rim, which is incised just anterior to the rim. A
sharp periosteal elevator lifts the periosteum off the rim, exposing
the entire floor of the orbit. Small malleable retractors are used
to dissect and retract, exposing the ethmoidal and naso-orbital areas. The floor of the orbit is thin and can usually be fractured with a small, curved
hemostat medial and lateral to the inferior neurovascular bundle. Alternatively, a
diamond burr may be used to create an opening in
the bony floor. Additional bone is removed with rongeurs, and care is
taken not to injure the infraorbital nerve and artery. Posteriorly, bone
is removed as completely as possible. We leave a strut of bone around
the infraorbital nerve anteriorly as a structure to support the globe
and prevent globe ptosis. Caution must be used near the orbital rim
in the inferomedial area to avoid injury to the nasolacrimal duct. Medially, the
ethmoids are removed to the frontoethmoidal suture superiorly
and the sphenoid sinus posteriorly. Front-biting rongeurs are used
to accomplish this; occasionally, digital pressure can be used to infracture
the posterior ethmoid sinuses and attain adequate medial decompressive
effect. Next, the periorbita is opened laterally, inferiorly, and medially to allow
orbital fat prolapse into the spaces created by the bone removal. We
use a scimitar-shaped blade for this; scissors may be used to enhance
or extend the incisions. Frequently, the fat is fibrotic and does
not prolapse spontaneously upon opening the periorbita. In this situation, portions
of fat may be gently teased through the incisions. The conjunctiva is closed with a running 6-0 Vicryl suture. The bone of
the lateral rim is replaced after the posterior portion is removed, and
holes are drilled to allow the rim to be tied into position with 4-0 nylon
suture. Some surgeons will secure the rim with titanium miniplates, and
others will not fix its position at all but will simply place
it in its original bed. The periorbita is closed over the bone with
interrupted 4-0 absorbable suture. This same material is used to close
the temporalis fascia. A 6-0 running plain catgut suture is used to close
the skin. A pressure dressing is placed over the eye and lateral
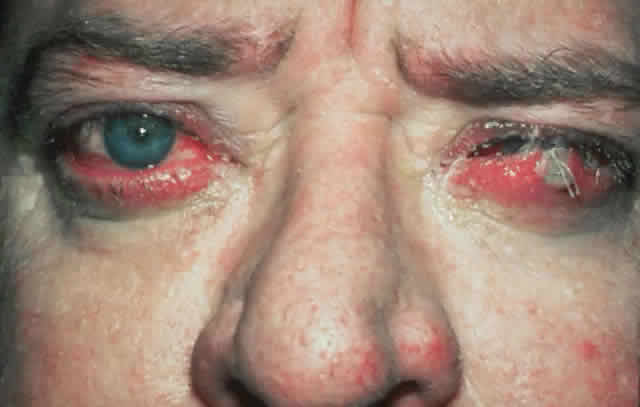
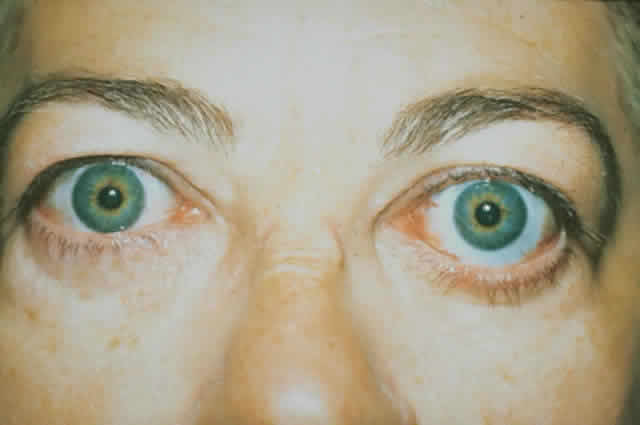
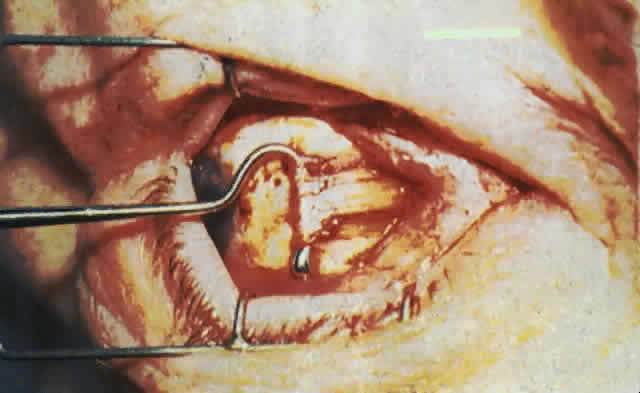
wall of the orbit (Fig. 17).  Fig. 17. A. A 38-year-old woman with marked dysthyroid proptosis and corneal exposure. Hertel
measurements right 31, left 30, base 101. B. Postoperative
appearance. Hertel right 20, left 18, base 101. Fig. 17. A. A 38-year-old woman with marked dysthyroid proptosis and corneal exposure. Hertel
measurements right 31, left 30, base 101. B. Postoperative
appearance. Hertel right 20, left 18, base 101.
|
The complications of orbital decompression can be significant and include, most
commonly, worsening of eyelid retraction or increased diplopia. Corneal
compromise can occur during or after surgery as well and can
sometimes be severe. The decompression can be inadequate or too generous, and
the ethmoid sinus or maxillary sinus can become infected postoperatively
on occasion. There have been injuries to the cribriform plate
where spinal fluid leaks from orbital decompression of the medial
wall and of the roof. Air can enter the dura directly, causing pneumocrania
on rare occasions, and death may ensue from complications of anesthesia
or, as in one case, a ruptured aneurysm of an unrelated anterior
communicating artery during this major surgery. The optic nerve can
be injured or devascularized during decompression and hemorrhage with
optic nerve compression, which can result in blindness. Orbital decompression
of any type is certainly a major procedure, and all complications
should be carefully explained to the patient and family before assuming
the risk of this type of surgery. |