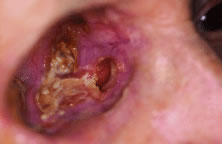
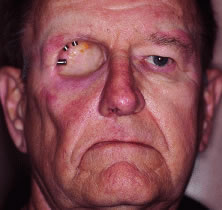
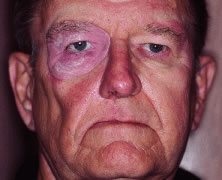
INDICATIONS FOR ENUCLEATION Recommending enucleation is one of the most difficult therapeutic decisions
in ophthalmic surgery. The most common indications for enucleation
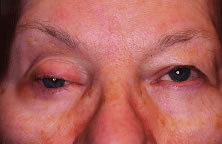
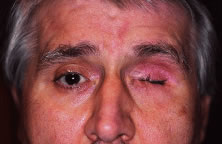
are treatment of intraocular malignancies, relief of pain in blind eyes; removal
of severely traumatized, deformed, or phthisical eyes without
visual potential; and prevention of sympathetic ophthalmia. Choroidal melanoma is the most common primary intraocular malignancy in
adults. Its treatment remains controversial and must be individualized
to each patient based on tumor size, tumor location, metastatic spread, and
patient preference. The mainstay of treatment has been enucleation.11 The Collaborative Ocular Melanoma Study (COMS) has investigated the role
of enucleation versus I-125 radioactive plaque therapy in medium-sized
tumors. The COMS also studied enucleation alone versus pre-enucleation
external beam radiation in eyes with large melanomas. The COMS found
that survival rates were similar in the large melanoma group. In 2003, the
COMS will have completed at least 5 years of follow-up for all
patients in the medium-sized tumor trial, and initial results regarding
survival and quality of life are anticipated at that time.12 The Zimmerman theory postulated that enucleation might induce metastatic
spread of the disease because of an increase in the intraocular pressure
during surgery. This theory was based on the findings in 1146 patients
with uveal melanomas that metastatic disease was found in only 1% of
patients preoperatively but increased to 8% during the second year
of follow-up.13 From information obtained in the COMS, there is evidence that enucleation
does not induce metastasis. Although this topic remains controversial, most
surgeons advocate gentle handling of the tissues, as well as
avoidance of opening the globe during enucleation surgery to decrease
the theoretical increased risk of tumor spread during enucleation surgery. Retinoblastoma is the most common intraocular malignancy of children. Although
other means of treatment exist such as chemotherapy, cryotherapy, radiation, and
photocoagulation, most eyes affected by retinoblastoma
are enucleated. Enucleation of the affected eye has resulted in survival
rates as high as 90%.14 In a review of 24,444 enucleation cases over a 55-year period, Spraul and
Grossniklaus15 found trauma to account for 40.9% of cases, whereas tumors were the cause
of enucleation in 24.2% of cases. Brackup and colleagues16 found that 34% of patients with open globe injuries eventually required
enucleation for pain control in a university referral practice. Custer17 reviewed 58 cases of enucleation for blind, painful eyes and found that 45% of
patients had sustained prior trauma. In the same study, 202 cases
of enucleation during a 15-year period were reviewed; intraocular
malignancy accounted for 54% of cases, blind painful eyes accounted for 33%, and
disfigured phthisical eyes for 6% of cases.17 Margo18 found that blind, painful eyes accounted for 43% of cases and was the
most common indication for enucleation in a study of enucleations in a
community hospital practice. The issue of primary enucleation must sometimes be addressed by any ophthalmologist
who cares for ocular trauma patients. In most cases, surgeons
initially try to salvage even severely traumatized eyes. Assessment
of visual acuity and obtaining proper informed consent may be impossible. There
is also some advantage in allowing the patient and the patient's
family to realize that the eye is no longer functional and
to come to terms emotionally with the loss of vision. Primary enucleation
is appropriate in certain circumstances. In patients who have experienced
explosive, thermal, or gunshot injuries to the eye, there is
typically no appreciable ocular tissue left to repair and primary enucleation
may be indicated. In other patients large corneoscleral lacerations
extending posterior to the ora serrata where no light perception (NLP) vision
is documented and prolapsed uveal and retinal tissue is
verified by frozen section, primary enucleation is a viable option. Of
course, complete examination to evaluate the status of the other eye
should be completed before surgery and detailed informed consent by the
patient or patient's guardian must be obtained. They must be made
well aware of the condition and realize that no chance of vision will
be possible in the future and that the eye will be removed. Typically associated with penetrating ocular trauma or surgery, sympathetic
ophthalmia is a rare, granulomatous panuveitis affecting both the
injured and uninjured eye. Characteristically, there is a diffuse lymphocytic
infiltration of the uveal tract with nonnecrotizing granulomas. The
precise cause and incidence is unknown. Most studies quote incidence
rates between 0.001% and 1.9% in traumatized eyes.19 Lubin and coworkers8 found that 65% of cases occurred between 2 weeks and 3 months following
the initial injury. Although the condition is extremely rare, enucleation
is the only known prophylaxis for sympathetic ophthalmia.19 Based on the previous information, enucleation has been recommended within 2 weeks
of the injury to prevent sympathetic ophthalmia. However, current
thoughts on the subject are under debate. Levin and coworkers4 undertook a chart review and survey of the members of the American Society
of Ophthalmic Plastic and Reconstructive Surgery, Uveitis Society, and
Eastern Ophthalmic Pathology Society in an effort to evaluate the
relationship between evisceration and sympathetic ophthalmia. First, of
the fifty-one patients who underwent evisceration at their institution, none
were found to have clinical evidence of sympathetic ophthalmia. The
survey portion of the study found no documented cases of sympathetic
ophthalmia among the respondents following evisceration. Less
that five cases of sympathetic ophthalmia were recalled but not documented
by the surveyed physicians. From this information, the authors concluded
that sympathetic ophthalmia is a rare disease and is infrequently
associated with evisceration. Levin and coworkers concluded that evisceration
is safe and effective procedure. It should be considered in
cases in which direct examination or ultrasound excludes intraocular
tumor, when there is adequate scleral volume, and when the pathologic
specimen is not important.4 THE ENUCLEATION PROCEDURE The most devastating surgical complication in ophthalmology would be the
removal of the incorrect eye. It is absolutely essential that the eye
to be removed is appropriately identified before surgery. Reviewing
the patient's chart and obtaining informed consent personally are
the first steps on the day of surgery. After the patient is asleep in
the operating room, verifying the correct eye by another chart review, as
well as direct examination of the patient, is essential. In patients
with intraocular tumors in which the eye typically appears normal
externally, a dilated examination is extremely helpful. I like to dilate
only the operative eye and identify the tumor just before draping the
patient. Marking the cornea of the operative eye with a surgical marking
pen also provides another safety check. Finally, a shield should
be placed over the nonoperative eye, and the patient should be prepped
and draped by the operating surgeon. These measures, although time consuming, are
important in preventing a disastrous situation. Enucleation is best performed under general anesthesia. After the patient
is asleep and has been prepped and draped by the surgeon, a retrobulbar
injection of 3 cc of a 50/50 mixture of 1% lidocaine and 0.5% bupivacaine
with 1:100,000 units of epinephrine is performed. An eyelid speculum
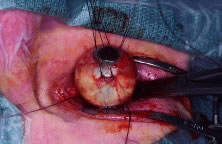
is placed, and a 360-degree limbal peritomy is performed with
blunt-tipped Westcott scissors and small toothed forceps. Using blunt-tipped
Steven's tenotomy scissors, the four quadrants between the
recti muscles are cleared (Fig. 4). This is performed by grasping the edge of conjunctiva and Tenon's
capsule, advancing the scissors posteriorly along the sclera to just
past the equator, and spreading the tissue by opening the scissors. Next, all
four recti muscles are identified, tagged with a double-armed 5-0 Vicryl
suture in a locking fashion, and cut free from the globe. Each
rectus muscle is identified and isolated on a muscle hook and excess
facial attachments are bluntly removed from the muscle's surface
with a cotton-tipped swab. One end of a double-armed 5-0 Vicryl is
then passed through the midportion of the muscle belly approximately 1 mm
from its insertion towards the edge of the muscle (Fig. 5). The suture is then passed through the underside of the muscle approximately 1 mm
from the muscle edge and pulled through so as to lock the
suture. The other end of the suture is passed similarly by starting the
pass where the first end started and exiting at the opposite muscle
edge. The sutures are then clamped or taped to the drapes. After all
four recti muscles have been removed from the globe, the superior oblique
muscle tendon is cut with Westcott scissors. Next, the inferior oblique
muscle is isolated with a muscle hook in the inferior medial quadrant, cauterized
across its belly, and cut (Fig. 6). A 4-0 silk suture is then placed in a running fashion through the remaining
muscle tissue of the medial and lateral recti muscles to be used
as traction sutures. While applying gentle upward traction, a large
hemostat is advanced towards the optic nerve, posterior to the globe, starting
at the lateral canthus. With the hemostat closed, the optic
nerve is identified. With the clamp superior to the nerve, gentle inferior
movement of the clamp will cause the eye to rotate superiorly. The
opposite will be observed with the hemostat inferior to the nerve. With
the position of the optic nerve accurately identified, the clamp is
opened, retracted slightly, advanced over the nerve, displaced a few
millimeters posteriorly, and closed. Again, gentle movement of the clamp
should verify that the nerve is securely clamped. With the nerve still
clamped, enucleation scissors are then advanced into the orbit, anterior
to the clamp, and the optic nerve is transected (Fig. 7). The globe is elevated with the traction sutures while any remaining
tissue adherent to the globe is cut with scissors. The socket is immediately
packed with two gauze sponges. During this time the orbital implant
can be wrapped with an appropriate wrapping material and prepared
for implantation. The globe is grossly examined and sent to the pathology
laboratory for examination. The packing is removed, the cut edge
of the optic nerve is isolated, and bipolar cautery is applied. The clamp
is carefully released under direct visualization to ensure that no
significant hemorrhage is present. The socket is then examined for any
sign of pathologic process. A small rent in posterior Tenon's capsule
corresponding to the opening for the optic nerve can be repaired
at this time using a single 5-0 Vicryl suture. Repair of this defect
in Tenon's capsule prevents unintentional posterior migration of
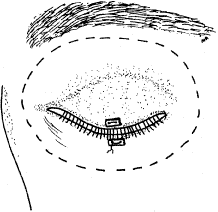
the orbital implant. 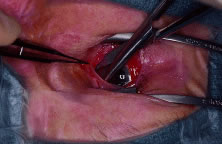 Fig. 4. Blunt-tipped scissors are placed in the inferior-nasal quadrant and opened
carefully while the conjunctiva and Tenon's capsule is retracted
superiorly. This action clears the quadrant of Tenon's tissue
and allows for isolation of the recti muscles. All four quadrants are
cleared in a similar fashion. Fig. 4. Blunt-tipped scissors are placed in the inferior-nasal quadrant and opened
carefully while the conjunctiva and Tenon's capsule is retracted
superiorly. This action clears the quadrant of Tenon's tissue
and allows for isolation of the recti muscles. All four quadrants are
cleared in a similar fashion.
|
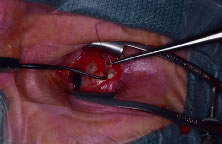 Fig. 5. A double-armed suture is place in the lateral rectus muscle. Fig. 5. A double-armed suture is place in the lateral rectus muscle.
|
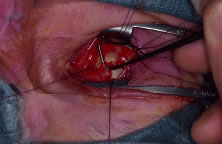 Fig. 6. The inferior oblique muscle is isolated on a muscle hook before cauterization
and transection. Fig. 6. The inferior oblique muscle is isolated on a muscle hook before cauterization
and transection.
|
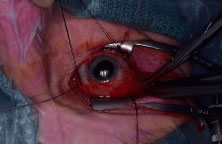 Fig. 7. After the optic nerve is clamped, curved enucleation scissors are used
to cut the optic nerve just anterior to the clamp. Fig. 7. After the optic nerve is clamped, curved enucleation scissors are used
to cut the optic nerve just anterior to the clamp.
|
In certain instances, it is appropriate to place the implant posterior
to Tenon's capsule, directly into the orbital fat. In these cases, placing
blunt-tipped scissors into the defect and spreading easily enlarges
the rent in posterior Tenon's capsule allowing for placement
of the implant directly into the muscle cone. To provide for the best possible prosthetic appearance and motility, an
implant with attached extraocular muscles should be placed within Tenon's
capsule. The largest implant that comfortably fits into the
socket should be used to decrease the risk of a superior sulcus deformity. Most
adults can accommodate a 20- or 22-mm implant without difficulty. Wrapping
an implant provides approximately an additional 2 mm of
diameter. (The average ocular diameter is 24 mm.) In general, the recti
muscles are attached to the implant directly or to the various materials
used to wrap orbital implants in a location corresponding to their
normal anatomic position. A complete discussion on the wide variety
of orbital implants and wrapping materials is found in the section on
implant and wrapping materials. A careful layered closure is mandatory
to decrease the risk of implant exposure. Tenon's capsule is closed
first with multiple interrupted 5-0 Vicryl sutures. Care should be
taken not to incorporate any conjunctiva into the deep closure. This
may allow for cyst formation, implant exposure, or extrusion. The conjunctiva
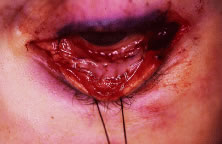
is then closed with a running suture of 7-0 Vicryl (Fig. 8). Antibiotic ointment and a conformer are then placed between the eyelids, and
the socket is pressure patched for 4 to 7 days. Some surgeons
place patients on prophylactic oral antibiotics for the first few days
following surgery. After the patch is removed, the patient is asked
to apply antibiotic ointment to the socket twice a day for the next 2 to 4 weeks. Continued
wear of the conformer is used to prevent shortening
of the conjunctival fornices. The patient is ready to see the ocularist 6 to 8 weeks
after surgery for the prosthesis fitting. Patients
should be encouraged to wear polycarbonate glasses to protect the remaining
eye. 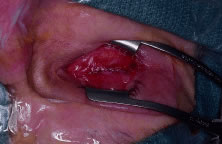 Fig. 8. The conjunctiva is closed with a running absorbable suture. Fig. 8. The conjunctiva is closed with a running absorbable suture.
|
INDICATIONS FOR EVISCERATION Evisceration is the removal of the ocular contents while leaving the sclera
and optic nerve and, in some cases, the cornea intact. Evisceration
offers several advantages over enucleation. Operating time is shorter, the
operation is less technically challenging, and the procedure can
be performed under retrobulbar anesthesia. There is less disruption
to the orbital tissues, better motility, and a more cosmetically acceptable
orbit when compared with enucleation. It is the preferred treatment
for endophthalmitis because it allows for extirpation and drainage
of the ocular contents without orbital invasion. In cases of endophthalmitis, orbital
implantation has typically taken place as a secondary
procedure. However, a recent study has found that primary implant placement
is a viable technique.20 Disadvantages include the theoretical risk of sympathetic ophthalmia and
a less complete specimen for pathologic examination to detect intraocular
malignancy or spread. Although controversy exists as to the exact
indications for evisceration and enucleation, evisceration should never
be performed in cases of suspected intraocular tumor. THE EVISCERATION PROCEDURE The appropriate eye for removal must be established as described previously. Unlike
enucleation, evisceration can be performed using a retrobulbar
injection and intravenous sedation. Whether monitored anesthesia
or general anesthesia is used, a retrobulbar injection of 3 cc of a 50/50 mixture
of 1% lidocaine and 0.5% bupivacaine with 1:100,000 units
of epinephrine is injected to control oozing and provide postoperative
pain control. The patient is then prepped and draped by the surgeon. An
eyelid speculum is placed and a 360-degree limbal peritomy is performed
with blunt-tipped Westcott scissors and small toothed forceps. Using
Steven's scissors, the four quadrants between the recti muscles
are cleared. This is performed by grasping the edge of conjunctiva
and Tenon's capsule, advancing the scissors posteriorly along the
sclera to just past the equator and spreading the tissue by opening
the scissors. Approximately 1 to 2 mm posterior to the limbus, a small full-thickness
scleral incision is made. Westcott scissors are then used to make a circumferential
incision around the globe to remove the cornea. If the
cornea is to be left in place, the incision is stopped just short of completion
leaving a small scleral hinge. The intraocular contents are
then separated from the sclera using an evisceration spoon or Freer periosteal
elevator. Bleeding from the optic nerve or penetrating vessels
can be controlled with gentle bipolar cautery. The pigment is meticulously
removed using absolute alcohol on a cotton-tipped applicator. The
scleral cavity is then copiously irrigated with antibiotic solution. Windows
oriented in an anterior to posterior direction are cut in the
sclera in the four quadrants between the recti muscles using scissors. The
sclera can also be opened around the optic nerve.21 These scleral windows allow for vascular ingrowth if a porous implant
is placed. Scissors are then used to make two cuts at the anterior opening
of the sclera in an inferior-medial and superior-lateral direction
to facilitate implant placement into the sclera. A sphere implant measuring
from 14 to 18 mm is placed into the scleral cavity (Fig. 9). Redundant sclera is trimmed and the sclera is closed with multiple interrupted 5-0 Mersiline
sutures. Tenon's capsule is closed first
with multiple interrupted 5-0 Vicryl sutures. The conjunctiva is then
closed with a running suture of 7-0 Vicryl (Fig. 10). Antibiotic ointment and a conformer are then placed between the eyelids, and
the socket is pressure patched for 4 to 7 days. Some surgeons
place patients on prophylactic oral antibiotics for the first few days
following surgery. After the patch is removed, the patient is asked
to apply antibiotic ointment to the socket twice a day for the next 2 to 4 weeks. Continued
wear of the conformer is essential to prevent shortening
of the conjunctival fornices. The patient is ready to see the
ocularist 6 to 8 weeks after surgery for the prosthesis fitting. As with
any monocular patient, polycarbonate glasses should be worn routinely
to protect the remaining eye. 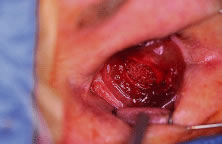 Fig. 9. A polyethylene spherical implant rests inside the scleral cavity before
closure. Fig. 9. A polyethylene spherical implant rests inside the scleral cavity before
closure.
|
 Fig. 10. The sclera, Tenon's capsule, and conjunctiva are closed in layers. Fig. 10. The sclera, Tenon's capsule, and conjunctiva are closed in layers.
|
IMPLANT AND WRAPPING MATERIALS Much of the interest in enucleation and evisceration surgery over the past
few years has concentrated on new types of orbital implant materials. Ideally, the
purpose of an orbital implant is to provide adequate
orbital volume to compensate for the absent globe, promote prosthesis
motility, and be responsible for minimal complications following surgery. Common
complications that have arisen include exposure, extrusion, infection, inflammation, and migration of the implant within the anophthalmic
socket. Since 1885 when Mules placed the first orbital implant consisting of a
blown glass sphere, various types of materials have been placed in the
orbit following removal of an eye.2 Glass, rubber, steel, gold, silver, silicone, acrylic, titanium mesh, and
polymethylmethacrylate (PMMA) spheres have been used (Fig. 11). Many of these materials are well tolerated by the host and provide adequate
orbital volume. However, direct extraocular muscle attachment
is impossible and motility is limited. Further refinement occurred with
the use of quasi-integrated implants such as the Allen, Iowa, and Universal
models. In these implants, the extraocular muscles were attached
the implants directly and implant movement was transferred to the prosthesis
via matching irregularly shaped surfaces on the implant and
prosthesis. Quasi-integrated implants are now rarely used because of their
difficulty of implantation and higher risk of migration and extrusion. 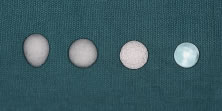 Fig. 11. From left to right: conical polyethylene, spherical polyethylene, hydroxyapatite, and
polymethylmethacrylate (PMMA) orbital implants. Fig. 11. From left to right: conical polyethylene, spherical polyethylene, hydroxyapatite, and
polymethylmethacrylate (PMMA) orbital implants.
|
In 1985, Perry introduced the use of a hydroxyapatite (HA) orbital implant
formed from a salt of calcium phosphate that is found in the mineralized
portion of human bone.22 The material is biocompatible, nontoxic, and nonallergenic. Its extensive
system of channels and pores facilitates fibrovascular ingrowth that
allows the implant to become vascularized and integrated into the orbital
tissues. The most common type of HA spheres (Bioeye; Integrated
Orbital Implants, Inc., San Diego, CA) currently used in the United States
are derived from sea coral. A synthetic HA is manufactured in France (FCI, Issy-Les-Moulineaux, France). A HA derivative derived from
the mineral portion of calf bone is available under the brand name M-sphere (IOP
Inc., Costa Mesa, CA). Although it has a long history of use
in other fields of surgery, the M-sphere is extremely fragile and requires
great care when using. A new bioceramic implant made of the material
Alumina (aluminum oxide) was approved for use in the United States
in early 2000. It represents a new type of porous integrated orbital
implant that is manufactured without disruption to marine ecosystems. Studies
are underway regarding its long-term success as an orbital implant. Because of the rigid nature of the material, HA implants must be wrapped
to facilitate attachment of the extraocular muscles. Donor sclera is
the most commonly used wrapping material. The main concern with the use
of donor sclera is the extremely low risk of disease transmission. Autologous
fascia or dermis can be used but harvesting the tissue can
greatly increase surgical time and leave undesirable scars. Other effective
wrapping materials producing similar results are now available on
the market. Processed human sclera (Tutoplast), bovine pericardium (Ocu-guard), and
acellular dermis are all appropriate wrapping materials. Some
surgeons have used polyglactin 910 (Vicryl) mesh as a wrapping
material. It has the advantage of being easy to use, extremely inexpensive, and
readily available with no risk of disease transmission.23 The use of Vicryl mesh is associated with an increased risk of exposure
unless the extraocular muscles are sutured anterior to their normal
anatomic position. This results in the implant being placed deeper into
the socket. Although polyglactin mesh facilitates implant insertion
and extraocular muscle attachment, it does not provide a permanent barrier
to exposure.17 Wrapping HA implants adds to the cost and complexity of the surgery. For
these and other reasons, the use of porous polyethylene implants (Medpor) were
investigated.24 Porous polyethylene has been used successfully in reconstructive surgery
for many years. It is biocompatible with channels that allow for vascularization
and integration similar to HA. However, porous polyethylene
implants have several advantages over HA. The material is less expensive
at approximately $400 compared with $650 for HA. It is soft enough
that the muscles can be directly attached to the material. It can
also be shaped and carved with a scalpel if needed. Some authors have
advocated truncation of the anterior surface of the implant to facilitate
prosthesis movement. Thus far, there is no indication that this improves
motility and may allow for implant deviation to become a problem.17 I prefer to use polyethylene (Medpor) as my implant of choice. I recommend
attaching an approximately 10- to 15-mm piece of sclera, fascia, periosteum, or
acellular dermis to the anterior surface of the porous
polyethylene implant with 5-0 Merseline suture (Fig. 12). However, some surgeons use polyethylene orbital implants without any
wrapping. I think it is easier to suture directly to the implant if has
been warmed for a few minutes in sterile saline. The implant is then
placed into Tenon's capsule, and the extraocular muscles are sewn
to the implant directly a few millimeters anterior to their normal
anatomic position. This corresponds to a position slightly anterior to
the edge of the piece of barrier tissue. I have also had excellent success
wrapping the anterior half of the implant with processed whole sclera (Tutoplast) or
donor sclera (Fig. 13). I make sure that the sclera is securely attached to the implant by using
multiple nonabsorbable 5-0 Merseline sutures. With this method, I
cut four windows in the sclera measuring 2 mm by 5 mm corresponding to
the positions of the extraocular muscle attachments. Each extraocular
muscle is then advanced through its respective window and sutured to
the sclera and implant. As with any implant material, Tenon's capsule
and conjunctiva are then meticulously closed in layers. 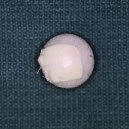 Fig. 12. A polyethylene sphere with a piece of sclera attached to its surface using
nonabsorbable suture. Fig. 12. A polyethylene sphere with a piece of sclera attached to its surface using
nonabsorbable suture.
|
 Fig. 13. A scleral-wrapped porous orbital implant. Windows to allow for attachment
of the extraocular muscles are visible. Fig. 13. A scleral-wrapped porous orbital implant. Windows to allow for attachment
of the extraocular muscles are visible.
|
Unlike alloplastic implants, extrusion and migration of HA integrated implants
is uncommon. The fibrovascular ingrowth prevents most exposed
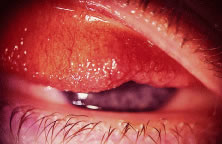
integrated implants from extruding.25 However, tissue breakdown and exposure of the anterior surface of the
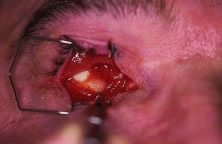
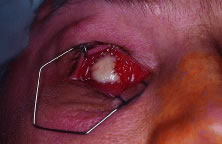
implant is reported to occur in 0% to 11.1% of cases25–27 (Fig. 14). Shields and coworkers28 reviewed 249 enucleation cases in which scleral-wrapped HA spheres were
placed and found an acceptable exposure rate of 1.6%. The risk of exposure
is reduced after vascularization of the anterior portion of the
implant. Exposure can be reduced with meticulous layered surgical closure, proper
implant size, drilling holes into the sphere before implantation, delayed
fitting of the prosthesis, and vaulting of the posterior
surface of the initial prosthesis to reduce pressure on the tissues
covering the anterior surface of the implant. 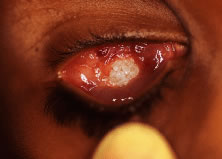 Fig. 14. An exposed polyethylene orbital implant. Fig. 14. An exposed polyethylene orbital implant.
|
Care must also be taken to avoid exposure problems when using porous polyethylene
implants as well. Karesh and Dresner24 first proposed the use of unwrapped porous polyethylene implants following
enucleation, evisceration, and secondary orbital implant surgery
with no signs of exposure in 21 patients. However, other investigators
have found exposure rates to be high ranging from 9% to 13%.29,30 Although some debate exists regarding the need to wrap porous polyethylene
implants, uncovered porous polyethylene implants appear to have a
complication rate higher that wrapped HA implants.17 Soparkar and coworkers31 studied the vascularization of porous polyethylene cubes placed in rabbits. From
the results of the study, they suggest an alteration in surgical
technique to improve and accelerate implant vascularization. They
advocate the use of a tissue barrier (sclera, fascia, and dermis) attached
to the anterior surface of the implant to prevent exposure but
believe that wrapping the implant is unnecessary. They also recommend
increasing the amount of muscle tissue in direct contact with the implant
by attaching the muscles anterior to their normal anatomic position, placing
posterior fixation sutures, and removing the muscle capsule
from the anterior 5 to 6 mm of each extraocular muscle. Currently, investigation
is also underway to determine if growth factors or angiogenesis-promoting
agents may enhance implant vascularization.32 Improved implant motility has always been a goal in implant design and
advancement. The motility of wrapped alloplastic and HA has been compared
in two studies.33,34 Both concluded that there is no motility benefit of nonpegged HA over
spherical alloplastic implants. Motility is due to the attachment of the
extraocular muscles to the wrapping and not to the implant material. Custer
and coworkers also determined that larger implants showed more
movement and that motility declines with advancing age. Other investigators
have shown that oval implants show no advantage over spherical
implants.30 Integrated implants might be unnecessary in patients who do not wish to
consider peg placement. In motivated patients desiring maximal motility, coupling pegs made of
titanium are available for use with both the HA and porous polyethylene
types of implants. The pegging systems are placed into the buried, vascularized
implant allowing direct coupling with the prosthesis. Before
peg placement, implant vascularization is typically confirmed 6 to 12 months
following surgery. This has been done in the past using bone
scan technology. Recent evidence has shown contrast-enhanced magnetic
resonance imaging (MRI) best suited for assessing the vascularization
of the porous polyethylene and HA implants.35 However, some investigators believe that titanium motility pegging systems
can be placed safely and effectively at the time of enucleation in
Medpor or HA implants.36,37 The current pegging system for HA implants supplied by Integrated Orbital
Implants, Inc. (San Diego, CA) consists of a threaded titanium sleeve
with a hollow core and various shapes and sizes of coupling pegs. The
pegging procedure is typically done under retrobulbar anesthesia and
sterile conditions. With the implant stabilized, a hole is first created
in the HA implant with progressively larger hypodermic needles. No
power drill is required. The threaded titanium screw is then advanced
into the implant with the aid of a sleeve driver until the top of the
sleeve is flush with the implant surface. A flat peg is placed into
the hollow core of the sleeve. The patient is treated with topical antibiotic
for a month during the healing process. The patient is then sent
to the ocularist to have the implant coupled to the prosthesis. The
ocularist will determine the best technique for coupling and will select
the appropriate titanium attachment. Complications such as peg extrusion, peg
loosening, chronic discharge, pyogenic granuloma, infection, and
conjunctival breakdown and exposure do arise from pegging. Jordan
and coworkers found 37.5% of patients who underwent the pegging procedure
in HA implants experienced problems. However, most cases used the
older polycarbonate pegging system. It is thought that the newer titanium
systems will have fewer complications. A pegging system is also available for use with Medpor implants. The motility
coupling post (Porex Surgical, Inc., College Park, GA) consists
of a threaded titanium peg that protrudes 4 mm above the implant surface (Figs. 15 and 16). Although the manufacturer recommends local anesthesia, some patients
may require a retrobulbar injection before the pegging procedure. The
conjunctiva is marked, and a small opening is made in the conjunctiva
using a disposable cautery. The implant is then stabilized with a provided
clamp. A hole is then carefully placed into the implant using a
manual drill bit attached to a screwdriver. The equipment is packaged
in a kit from the manufacturer. It is important to maintain the appropriate
orientation of the drill, which is perpendicular to the implant, while
making the initial hole. The peg is then twisted into placed using
a special screwdriver until the threads are no longer visible. A conformer
and antibiotic ointment is then placed. At approximately 1 month
after pegging, the patient is ready for coupling with the prosthesis
by the ocularist. Reported complications associated with the motility
coupling post are uncommon but include pyogenic granuloma and conjunctival
overgrowth.  Fig. 15. The threaded peg used with Medpor implants is shown next to a surgical
ruler. Fig. 15. The threaded peg used with Medpor implants is shown next to a surgical
ruler.
|
 Fig. 16. Although the peg is typically placed after orbital implant vascularization
within the socket, this photo shows how the peg extends from the implant. Fig. 16. Although the peg is typically placed after orbital implant vascularization
within the socket, this photo shows how the peg extends from the implant.
|
When pegging either the HA or polyethylene implants, preoperative planning
with the ocularist is very helpful in determining the best position
of the peg. |