In certain instances discussed later in this chapter, it is not desirable to insert a synthetic implant within the orbit. In these cases, a dermal fat graft may be used.
The nonvascularizing, vascularizing, and dermal fat graft implants are also used for secondary intraorbital implantation.
BASIC ENUCLEATION SURGICAL TECHNIQUE
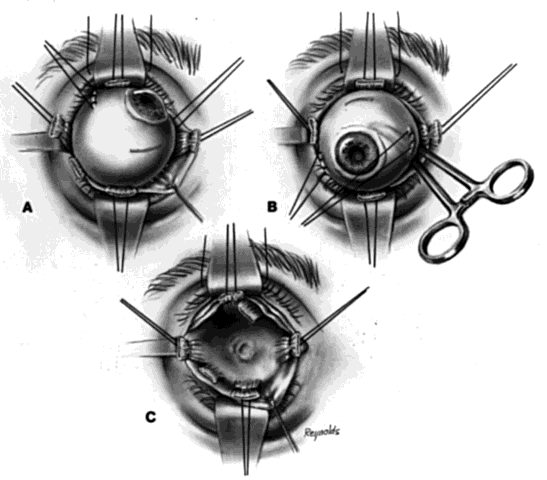
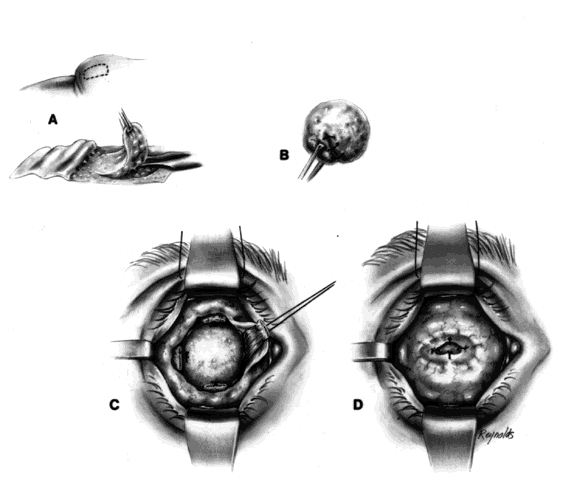
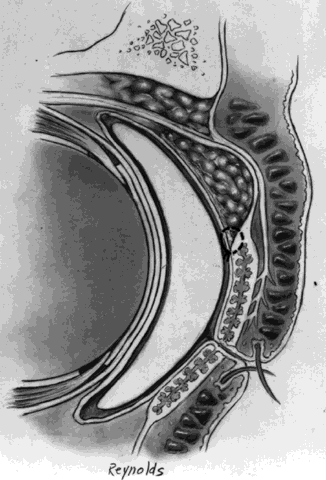
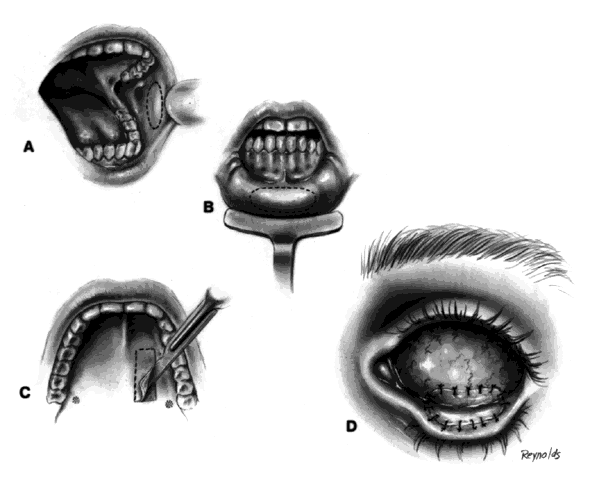
The basic enucleation technique, irrespective of the type of implant used, consists of removal of the diseased globe (Fig. 1).
Either general or local anesthesia may be used. General anesthesia is preferred because of the psychological implications of an enucleation. Preoperative in-depth discussion with the patient regarding what to expect postoperatively and what type of cosmetic result to expect is essential.
Regardless of whether general or local anesthesia is used, a 5-mL retrobulbar anesthetic injection should be given to eliminate the vagal reflex that can occur when traction is placed on the extraocular muscles or globe.
An eyelid speculum is inserted and a superior fornix double-armed 4-0 suture is hung by a hemostat. This aids in retracting the levator muscle complex and helps prevent injury to these structures.
Using a 30-gauge needle, an anesthetic solution or a balanced salt solution is injected subconjunctivally for 360°. This allows the surgeon to carefully perform a 360° peritomy, keeping the dissection in the episcleral plane and extending it to the four extraocular rectus muscles.
Each rectus muscle is severed at its insertion, and the tendons are tagged with a 5-0 or 6-0 synthetic absorbable suture, such as Vicryl or Dexon. The oblique muscles are not tagged but rather are severed close to their insertions on the globe and allowed to retract.
Two 4-0 black silk sutures are placed through the medial and lateral rectus muscle stumps on the globe. These sutures are then used as traction sutures for elevating and proptosing the globe. At this time, as many fibrinous connections as possible between Tenon's fascia and the episclera should be severed. An Arruga or similar type of speculum is useful at this stage.
This next step prevents significant bleeding once the optic nerve is severed. An Arruga speculum or a comparable speculum is placed between the medial rectus muscle and the globe. The medial and lateral traction sutures pull up on the globe, and a curved hemostat is used to clamp the optic nerve and the surrounding arteries. This hemostat clamp is left in place for 30 to 60 seconds and then removed. Enucleation scissors are used to sever the optic nerve. The globe is now removed. Any fine, residual fibrinous strands are cut. There usually is some bleeding, and the socket may be packed with a 4 × 4 gauze pad soaked with balanced salt solution. Occasionally, an epinephrine pledget is required when placing pressure against the posterior orbital structures.
I do not like or advocate the use of a snare, because when a snare severs the optic nerve, orbital fat is often snared and removed also. In addition, use of a snare is somewhat of a blind procedure, and occasionally, especially in an eye with a staphyloma, the posterior portion of the globe may be severed.
The socket should now be clean and dry, and a small rent should be visible posteriorly where the optic nerve went through the posterior Tenon's fascia.
A decision is now made as to the type of implant to use and its placement (i.e., within Tenon's fascia or posterior to the posterior layer of Tenon's fascia, within the muscle cone). Tenon's fascia that is posterior to the exits of the four rectus muscles is known as posterior Tenon's fascia and, as described previously, is thinner than anterior Tenon's fascia, which lies between the exits of the extraocular muscles and the conjunctiva. In general, the decision is made preoperatively as to whether or not a dermal fat graft is indicated. The technique and the implant used determine the immediate and long-term postoperative results.
INSERTION OF A HYDROXYAPATITE IMPLANT WITHIN TENON'S FASCIA
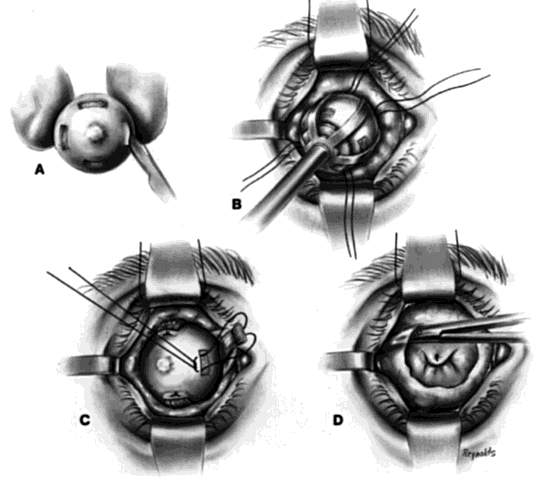
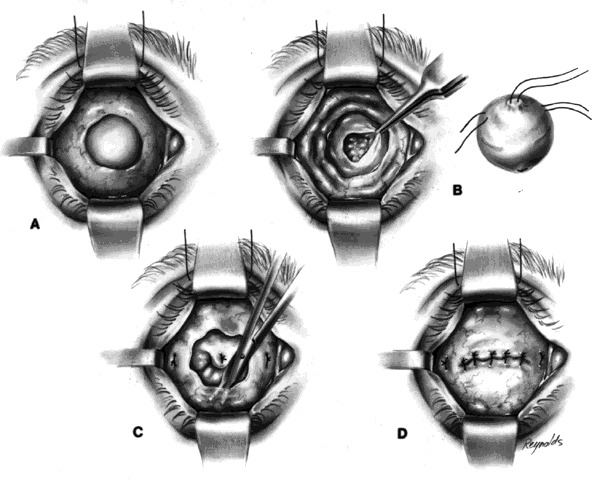
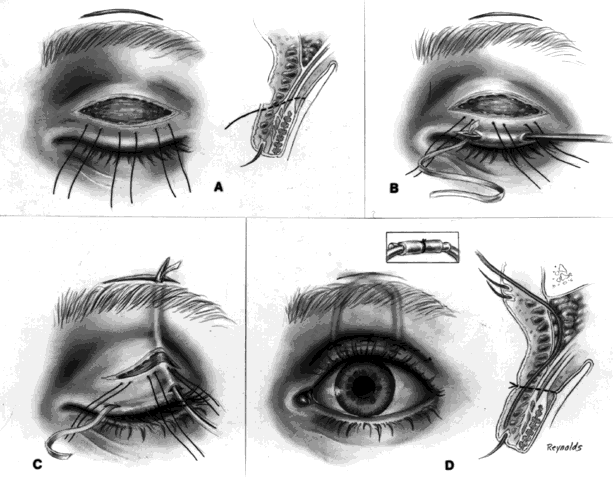
This technique is most useful when inserting a vascularizing implant, such as a hydroxyapatite implant (Fig. 2).8 Hydroxyapatite has a porous microarchitecture similar to human cancellous bone. Once implanted in an orbit with surrounding vascularized tissue, the implant readily fills with vascular tissue and becomes what might be termed as a living part of the orbit. This unique characteristic of a hydroxyapatite implant allows for the drilling of a hole through the anterior surface of the implant, once complete healing and vascularization of the implant have taken place. The walls of this drill hole become lined with conjunctival epithelium and allow for the insertion of a coupling peg, which aids in movement of the prosthesis. The surgeon evaluates the size of the hydroxyapatite implant to be used by first inserting sizing spheres. The properly sized sterile hydroxyapatite implant is wrapped in a preserved scleral shell. Autogenous temporalis fascia or fascia lata also may be used. The implant is wrapped to facilitate attachment of the extraocular muscles to the implant. Sclera preserved by freezing or by 95% alcohol preservation may be used. If preserved in 95% alcohol, the sclera should be washed thoroughly by three or more consecutive 2- to 3-hour washes with balanced salt solution. The scleral-wrapped implant may then be soaked in an antibiotic solution. The scleral envelope may not easily fit over the implant. If this is the case, then relaxing incisions must be made and resutured with either 5-0 Vicryl, Dexon, or Dacron sutures. The posterior portion of the implant should be exposed to facilitate vascularization.
In most instances, the size of the hydroxyapatite implant varies between 18 and 20 mm in diameter. When wrapped with sclera, the diameter of an implant increases by about 1.5 mm. An average eye 24 mm in axial length has a volume of 7.2 mL. The average prosthesis has a volume of 2.5 mL. Thus, to fill the volume replacement of a 24-mm globe, one would need an intraorbital implant volume of 4.7 mL (7.2 mL - 2.5 mL = 4.7 mL). Table 1 indicates that in this instance, a wrapped implant of 19.5 mm or an unwrapped implant of 21 mm would be required.
TABLE 1. Measurements for Proper Fit of a Hydroxyapatite Ocular Implant
| Diameter of Enucleated Globe | Volume of Enucleated Globe | Volume of Artificial Eye | Net Volume to be Replaced | Size of Implant if Not Wrapped | Size of Implant to Be Wrapped |
| (mm) | (mL) | (mL) | (mL) | (mm) | (mm) |
| 20 | 4.2 | 2.5 | 1.7 | 15 | 13.5 |
| 21 | 4.9 | 2.5 | 2.4 | 16.5 | 15 |
| 22 | 5.6 | 2.5 | 3.1 | 18 | 16.5 |
| 23 | 6.4 | 2.5 | 3.9 | 19.5 | 18 |
| 24 | 7.2 | 2.5 | 4.7 | 21 | 19.5 |
| 25 | 8.2 | 2.5 | 5.7 | 22 | 20.5 |
| 26 | 9.2 | 2.5 | 6.7 | 23 | 22 |
The enophthalmic appearance found in many anophthalmic orbits occurred as a result of volume deficiencies secondary to less volume of the implant and prosthesis as compared with the volume of the normal eye, and also because of some additional atrophy of orbital fat.
One may insert a bare hydroxyapatite implant by wrapping it in plastic strips and removing the strips once the implant is in position. It is difficult to place a bare hydroxyapatite implant because of spicules protruding from the surface of the implant, which tend to catch on tissue. If a bare implant is inserted, the rest of the technique is similar to the technique used for insertion of nonvascularizing implants. In this situation, the posterior rent in Tenon's fascia is sutured, and the sutures attached to the four extraocular muscle stumps are brought out through the conjunctival fornices because they cannot be attached directly to the bare hydroxyapatite implant.
If the implant is scleral-wrapped or fascial-wrapped, it is marked in four quadrants with a marking pen in areas where the four rectus muscles are to be attached. Windows in the sclera or fascia, approximately 4 mm wide and 7 to 8 mm long, are cut in the covering tissue, and the double-armed sutures previously attached to each extraocular muscle are brought out through the anterior scleral or fascial edge of the windows and tied. The implant is now firmly within Tenon's fascia with the posterior surface of the implant exposed to tissue within the muscle cone, because the rent in posterior Tenon's fascia, where the optic nerve passed through, is not sutured when the hydroxyapatite implant wrapped in sclera or fascia is used.
Vascularization of the implant occurs early on by blood vessels invading the hydroxyapatite through the posterior exposed areas and also through the windows through which the extraocular muscles were attached.
Some surgeons drill holes in the hydroxyapatite implant to facilitate vascularization; I have not found this to be necessary.
The conjunctiva is separated from the underlying anterior Tenon's fascia by injection of a balanced salt solution or an anesthetic solution between the two, with undermining to the medial, inferior, and lateral bony orbits, and superiorly as high as possible without affecting the levator muscle complex. This anterior layer of Tenon's fascia is imbricated and closed with 5-0 Vicryl or Dexon sutures. The conjunctiva is closed with either interrupted or 5-0 plain gut sutures. It is essential that the fornices be deep. A conformer is placed between the eyelids. The socket is dressed with an antibiotic solution, and a mild pressure bandage is applied for a period of 5 to 6 days. An artificial eye may be fit after 4 to 6 weeks.
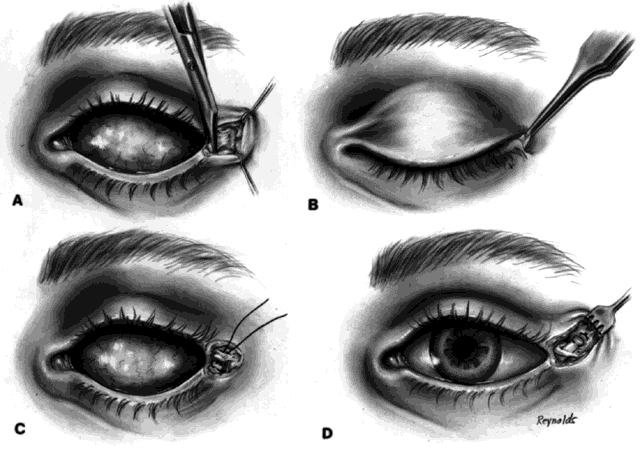
After 4 to 6 months, the implant should be vascularized. This may be confirmed with a technetium 99m bone scan or with a magnetic resonance imaging (MRI) scan with contrast. The bone scan should show evidence of vascularization at a level between that seen in normal orbital bones and that seen in midfacial bones. If the patient is anxious for increased motility when the implant is determined to be fully vascularized (in my experience this occurs only in approximately 30% to 40% of patients), then a peg hole can be drilled in the implant.
A retrobulbar anesthetic injection is given. The center of the orbital implant should be drilled in a perpendicular manner with a 3-mm cutting blade to a depth of 10 to 12 mm. A flat-topped peg is then inserted. The artificial eye is next replaced in the socket and antibiotic drops are used for a period of 4 to 6 weeks, after which time the drill hole is usually completely lined with conjunctival epithelium. The flat peg is then replaced with a ball-type peg, and the ocularist burrs a corresponding socket in the appropriate part of the posterior portion of the prosthesis so that a coupling of the implant and prosthesis occurs. This then becomes a truly coupled implant and prosthesis (Fig. 3).
This was never possible in the past because synthetic pegs attached to implants, exited through conjunctiva, and acted as a nidus for infection to enter the orbit. These early so-called integrated implants were disasters and were followed by the development of contracted sockets. This problem does not occur with hydroxyapatite implants because there is no exposure of the implant and the peg is in a cavity lined with conjunctival epithelium.
MANAGEMENT OF COMPLICATIONS RELATED TO HYDROXYAPATITE IMPLANTS
Because a hydroxyapatite implant becomes an integral part of the orbit by virtue of invasion of blood vessels and fibrous tissue within the implant, postoperative complications are handled in a specific manner. Exposure of the implant surface is not common, but it has occurred. It has been my experience that this problem cannot be satisfactorily managed by undermining and advancing conjunctiva and Tenon's fascia over the defect. If the defect is small and secondary to a poorly fitting prosthesis, and if the fit of the prosthesis is corrected, the defect may granulate in and re-epithelialize. As in all anophthalmic sockets, it is essential to have a properly fitting prosthesis. Most often, however, the exposure is great when the patient presents in the ophthalmologist's office. In this instance, my procedure of choice is to undermine conjunctiva and Tenon's fascia over the implant, making no attempt to significantly advance these tissues. A de-epithelialized dermal graft is placed over the exposed implant surface, the edges of the graft are tucked under the adjacent conjunctiva, and sutured with 6-0 Vicryl or Dexon sutures. The dermal graft should extend several millimeters under the adjacent Tenon's fascia and conjunctival tissue. The graft vascularizes and re-epithelializes with conjunctival epithelium. After 4 to 6 weeks, a properly fitting artificial eye is given to the patient. It is also possible to use this technique with autogenous fascia lata, temporalis fascia, or donor sclera.
Other problems unique to the hydroxyapatite implant are related to the peg. The shaft of the peg must be perpendicular to the apex of the socket and the implant. If the shaft is drilled at an angle, there is a great likelihood that extrusion of the peg will occur. This problem is less common but does occur, even in patients in whom the hole for the peg shaft has been drilled properly. When this occurs, the shaft hole becomes filled with fibrous tissue, which extrudes the shaft of the peg. Once the peg is out, the surface of the implant becomes resurfaced with conjunctiva and the patient may again be fitted with a standard artificial eye. If desired by the patient, the ophthalmologist may drill a repeat hole. Often, however, the patient is satisfied with the motility, and nothing further need be done.
A less common complication is the erosion of a spicule of hydroxyapatite through the conjunctiva at the start of or in the depths of the drill hole. The offending spicule should be burred off.
Because this problem has occurred even in properly drilled shafts, Perry and his co-workers have devised a plastic screw mechanism that is threaded into the implant at the time of drilling.8 This screw implant has a central shaft into which the peg is fit. Early results have indicated that this mechanism is more satisfactory than merely drilling the hole for the shaft of the peg directly into the implant.
INSERTION OF A NONVASCULARIZED IMPLANT
Nonvascularized implants such as polymethylmethacrylate or silicone (scleral- or fascial-wrapped, or without any wrapping) may be inserted within Tenon's fascia or posterior to all layers of Tenon's fascia within the muscle cone. If inserted within Tenon's fascia, the technique is as previously described; however, the posterior opening in Tenon's fascia where the optic nerve originally passed through should first be closed to prevent migration of the sphere. The extraocular muscles should never be imbricated over the anterior surface of the implant because this causes displacement and migration of the implant. Rather, they should be attached to fornix tissue by bringing the suture ends full-thickness out through the fornices and tying them over the conjunctival surface.
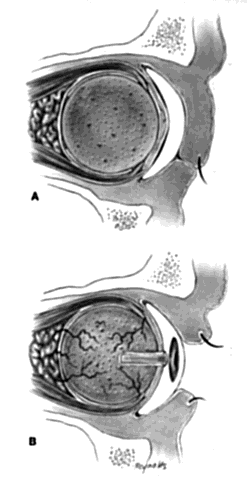
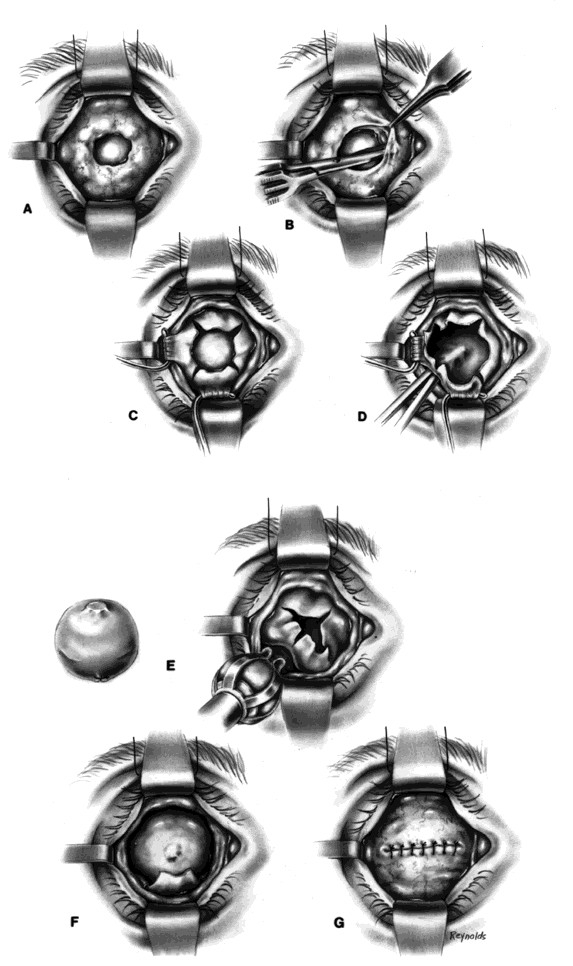
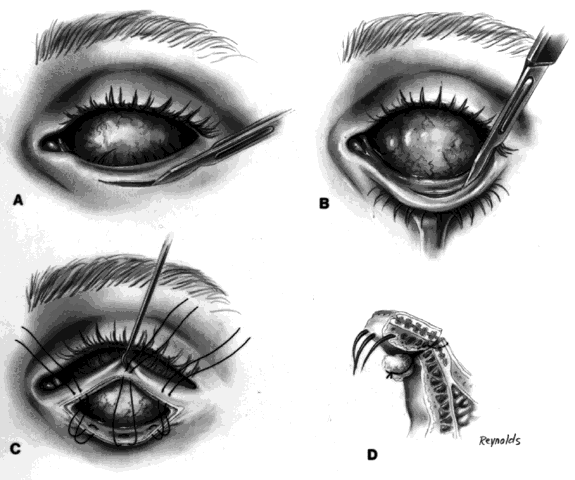
My preference when using these type of implants is to insert the nonvascularized scleral- or fascial-covered implant posterior to all layers of Tenon's fascia within the muscle cone. A larger implant can be used in this space, and there is little, if any, tendency for migration (Fig. 4).9,10
Ideally, the implant is wrapped in sclera or fascia, and three 5-0 or 6-0 double-armed Vicryl or Dexon sutures are attached to this scleral or fascial shell. Two sutures are placed where the medial and lateral rectus muscles would ordinarily be. These sutures are brought out through posterior Tenon's fascia medially and laterally and tied. A central, double-armed apical suture, passed through the scleral or fascial covering of the implant, is then used to imbricate posterior Tenon's fascia over the implant. Additional sutures may also be used to close any openings in posterior Tenon's fascia. These sutures virtually lock the implant in position. The superior and inferior rectus muscles are allowed to retract. They still continue to function. Pulling the superior rectus muscle forward can cause ptosis postoperatively because of its attachments to the levator muscle. Anterior Tenon's fascia is then separated from conjunctiva and undermined to the medial, inferior, and lateral orbital walls, and superiorly as high as possible without injuring the levator muscle complex. The double-armed sutures that were attached to the medial and lateral extraocular muscle tendons are now brought out full-thickness through anterior Tenon's fascia and conjunctiva and tied in the respective conjunctival fornices. Anterior Tenon's fascia is imbricated with use of 4-0 or 5-0 Vicryl or Dexon mattress sutures. The conjunctiva is closed with interrupted sutures or a running 6-0 plain gut suture.
It should be mentioned at this time that the undermining and separating of anterior Tenon's fascia and conjunctiva is essential to create deep fornices. It is the fornices that allow for movement of the prosthesis. For example, in a patient with a right-sided prosthesis, if the patient looks to the right, the lateral fornix deepens, the medial fornix shallows, and the prosthesis is literally pushed toward the right and drops into the space created by the deeper lateral fornix. The same type of situation occurs in whatever direction the patient looks. In the situation described, if the right lateral fornix were shallow, the prosthesis would be restricted in lateral motion.
Complications Associated With Nonvascularized Orbital Implants
Exposure, extrusion, and migration of the implants are complications related to these type of implants. If the exposure is small, undermining and advancing conjunctiva and Tenon's fascia may be all that is necessary for its correction. Late exposure is often due to an improperly fitting prosthesis, which causes pressure on the posterior socket wall. For large exposures, insertion of a scleral transplant or an autogenous de-epithelialized dermal transplant, as previously described, is usually satisfactory. If the exposure is very large and extrusion is imminent, it is usually best to remove the implant and insert a secondary implant within the muscle cone or to do a dermal fat graft.11–13
DERMAL FAT GRAFTS
There are situations in which placement of an intraorbital implant is not advisable but orbital bulk is necessary to fill the void left by the enucleated globe. This situation is not uncommon after extreme trauma when Tenon's fascia is severely lacerated. Also, this type of implant often does well in irradiated orbits where healing of tissue over synthetic implants may be a problem.
Surgical Technique
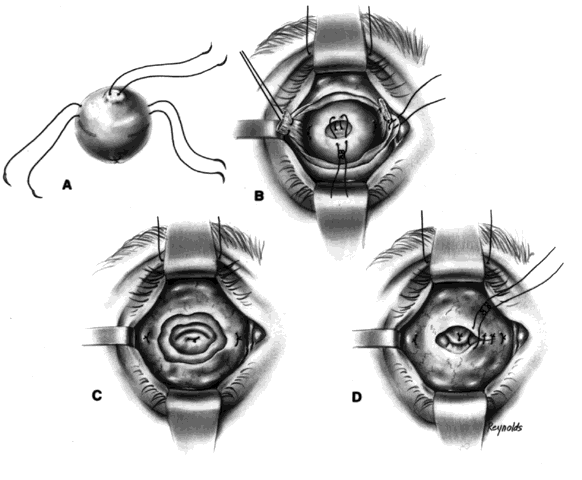
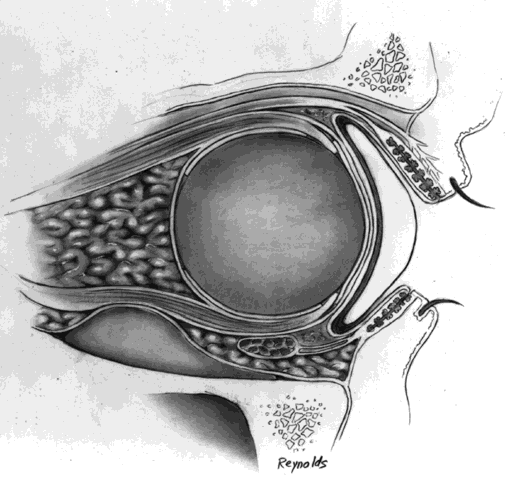
The globe is removed in the manner previously described. A de-epithelialized dermal fat graft is taken from the lateral buttock area; usually a graft 20 mm in diameter is taken. The donor site is closed with 2-0 Vicryl or Dexon sutures. If a 20/1000-in split-thickness skin flap was elevated first, this may be replaced with 6-0 Vicryl or Dexon sutures over the sutured donor site (Fig. 5).
The de-epithelialized dermal graft is sutured into the orbit with the four extraocular muscles and Tenon's fascia sutured to the dermis. To decrease the amount of fat atrophy, it is advisable to encase the donor fat within a dermal shell, essentially making a baseball-type of implant. The sutures used to close dermal edges to dermal edges can be either 4-0 or 5-0 Vicryl or Dexon sutures. Conjunctival edges are sutured directly to the dermis. It is not necessary to completely cover the entire dermal graft with conjunctiva if there is a conjunctival deficiency. The central surface of the dermal graft will usually re-epithelialize if the defect is no larger than 5 to 6 mm.
It is my experience that if the extraocular muscles are not readily identifiable, as may occur in a secondary dermal fat implantation, the dermal fat graft can be sutured to Tenon's fascia. Movement of the prosthesis still occurs because of the connections of the extraocular muscles to the Tenon's fascial sheaths, as previously described in the section on Anatomic Considerations.
The socket is dressed with an antibiotic solution or ointment, and a conformer and patch are left in place for 5 days. After this time, an antibiotic ointment is applied daily for a period of 4 to 6 weeks. The patient now is ready for an artificial eye.
Complications of Dermal Fat Grafts
If the orbital circulation is poor, the central apical portion of the graft may necrose. In most instances, this area eventually granulates in. The only treatment necessary is the use of a topical antibiotic ointment to prevent infection. The defect usually granulates in without a problem.
If the orbital circulation is extremely poor, it may not be able to support the dermal fat graft, and necrosis of the entire graft is possible. The result is a contracted socket, the repair of which is discussed later.
If the orbital circulation is so poor that it does not even support a dermal fat graft, then the problem should be addressed first by use of a temporalis muscle transfer from the anterior portion of the temporalis muscle into the orbit through an osteotomy in the lateral orbital wall.
EVISCERATION
By definition, this is the removal of the contents of the globe, leaving the scleral shell. The surgical procedure may be performed with preservation or excision of the cornea.
Evisceration as a primary procedure is less popular today than it was in the past. The main indication for evisceration is an endophthalmitis, either acute with no response to therapy, or old with loss of any vision in the affected globe. Most of these cases are secondary to cataract surgery; however, endophthalmitis can be metastatic and can occur secondary to intraocular foreign bodies. It can also occur secondary to perforation of a very virulent corneal ulcer.
When an evisceration is performed for endophthalmitis secondary to cataract surgery, and a large limbal incision was originally used, it is best to excise the cornea, insert the secondary implant, imbricate the scleral edges, and cover the area with conjunctiva. This type of evisceration should be performed whenever there is compromise of limbal or corneal integrity. I frequently insert an antibiotic dusting powder within the scleral shell, which previously was wiped very clean with 70% alcohol and irrigated with balanced salt solution. The contents of the globe in this instance are scooped out with an evisceration spoon or a cyclodialysis spatula. If the cornea is to be preserved, an incision is made between the superior and lateral rectus muscles parallel with the limbus. The incision is carried down to the choroid. A cyclodialysis spatula is then used to break all connections between the choroid and the sclera and also to transsect the optic nerve fibers. The incision is then enlarged so that an intrascleral implant can be inserted. The scleral edges are imbricated and sutured with multiple 4-0 or 5-0 mattress sutures and the conjunctiva closed. The only variation is when a vascular type of implant such as hydroxyapatite is inserted within the scleral shell. In this instance, posterior windows of sclera should be excised to allow vascular tissue to invade the implant. If a vascularized implant is used, a peg can be drilled similar to the way the peg was drilled in the enucleated socket. If the cornea was preserved, it is not necessary to bring the conjunctiva over the corneal surface.
Traditionally, eviscerations have been thought to give better cosmetic results than enucleations. However, with current enucleation techniques, the results are very comparable.