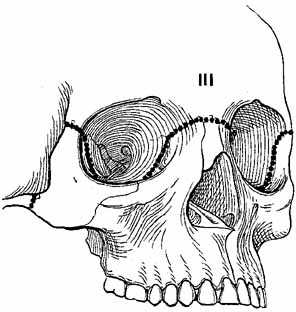
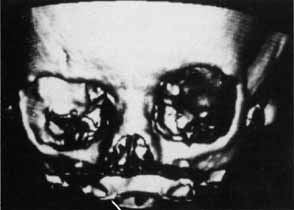
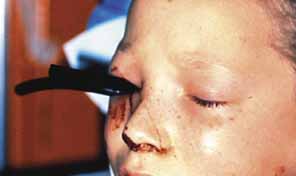
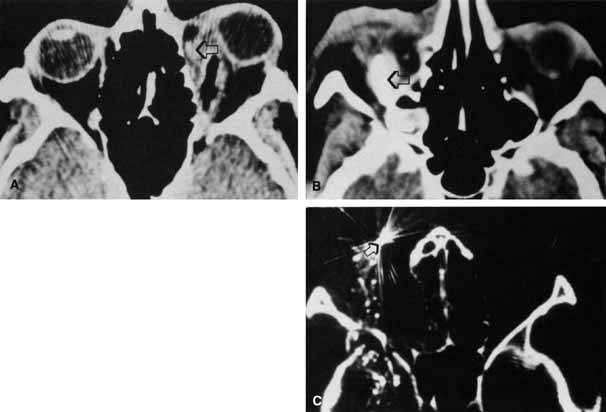
1. Holt JE, Holt GR, Blodgett JM: Ocular injuries sustained during blunt facial trauma. Ophthalmology 90:14, 1983 2. Klopfer J, Tielsch M, Vitale S, et al: Ocular trauma in the United States: Eye injuries resulting in hospitalization, 1984 through 1987. Arch Ophthalmol 110:838, 1992 3. Hughes B: Indirect injury of the optic nerves and chiasm. Bull Johns Hopkins Hosp 111:98, 1962 4. Miller NR: The management of traumatic optic neuropathy. Arch Ophthalmol 108:1086, 1990 5. Kennerdell JA, Amsbaugh CA, Myers EN: Transantral-ethmoidal decompression of optic canal fracture. Arch Ophthalmol 94:1040, 1976 6. Anderson RL, Panje WR, Gross CE: Optic nerve blindness following blunt forehead trauma. Ophthalmology 89:445, 1982 7. Gross C, DeKock J, Panje W, et al: Evidence for orbital deformation that may contribute to monocular blindness
following minor frontal head trauma. J Neurosurg 55:963, 1981 8. Frenkel REP, Spoor TC: Diagnosis and management of traumatic optic neuropathies. Adv Ophthal Plast Reconstr Surg 6:71, 1987 9. Kline LB, Morawetz RB, Swaid NS: Indirect injury of the optic nerve. Neurosurgery 14:756, 1984 10. Steinsapir KG, Goldberg RA: Traumatic optic neuropathy. Surv Ophthalmol 38:487, 1994 11. Wolin MJ, Lavin PJM: Spontaneous visual recovery from traumatic optic neuropathy after blunt
head injury. Am J Ophthalmol 109:430, 1990 12. Lessell S: Indirect optic nerve trauma. Arch Ophthalmol 107:382, 1989 13. Spoor TC, Hartel WC, Lensink DB, Wilkinson MJ: Treatment of traumatic optic neuropathy with corticosteroids. Am J Ophthalmol 110:665, 1990 14. Sieff SR: High dose corticosteroids for treatment of vision loss due to indirect
injury to the optic nerve. Ophthalmic Surg 21:389–395, 1990 15. Seiff SR: Trauma and the optic nerve. Ophthalmol Clin N Am 5:389–394, 1992 16. Demopoulos HB, Flamm ES, Pietronigro DD, et al: The free radical pathology and the microcirculation in the major central
nervous system disorders. Acta Physiol Scand 492:91–119, 1980 17. Demopoulos HB, Flamm ES, Seligman ML, et al: Further studies on free-radical pathology in the major central nervous
system disorders: Effect of very high doses of methylprednisolone
on the functional outcome, morphology, and chemistry of experimental
spinal cord impact injury. Can J Physiol Pharmacol 60:1415–1424, 1982 18. Bracken MB, Holford TR: Effects of timing of methylprednisolone or naloxone administration on recovery
of segmental and long-tract neurological function in NASCIS2. J Neurosurg 79:500–503, 1993 19. Fujitani T, Inoue K, Takahashi T, et al: Indirect traumatic optic neuropathy: Visual outcome of operative and non-operative
cases. Jpn J Ophthalmol 30:125, 1986 20. Spoor TC: Penetrating orbital injuries. Adv Ophthal Plast Reconstr Surg 7:193, 1988 21. Niho S, Niho M, Niho K: Decompression of the optic canal by the transethmoidal route and decompression
of the superior orbital fissure. Can J Ophthalmol 5:22, 1970 22. Fukado Y: Results of 350 cases of surgical decompression of the optic nerve. Trans Ophthalmol Soc N Z 25:96, 1975 23. Fukado Y: Results in 400 cases of surgical decompression of the optic nerve. Mod Probl Ophthalmol 14:474, 1975 24. Joseph MP, Lessell S, Rizzo J, Momose KJ: Extracranial optic nerve decompression for traumatic optic neuropathy. Arch Ophthalmol 108:1091, 1990 25. Call NB: Decompression of the optic nerve in the optic canal. Ophthal Plast Reconstr Surg 2:133, 1986 26. Maroon JC, Kennerdell JS: Surgical approaches to the orbit: Indications and techniques. J Neurosurg 60:1226, 1984 27. Gioia VM, Linberg JV, McCormick SA: Anatomy of the lateral canthal tendon. Arch Ophthalmol 105:529, 1987 28. Smith B, Eegan WFJr : Blow-out fracture of the orbit: Mechanism and correction of internal
orbital fracture. Am J Ophthalmol 44:733, 1957 29. Fujino T, Makino K: Entrapment mechanisms and ocular injury in orbital blow-out fractures. Plast Reconstr Surg 65:571, 1980 30. Jordan DR, Allen LH, White J, et al: Intervention within days for some orbital floor fractures: The white-eyed
blowout. Ophthal Plast Reconstr Surg 14:379–390, 1998 31. Burstine MA: Clinical recommendations for repair of isolated orbital floor fractures. An
evidence-based analysis. Ophthalmol 109:1207, 2002 32. Dutton JJ: Management of blow-out fractures of the orbital floor. I. Editorial. Surv Ophthalmol 35:279, 1991 33. Manson PN, Iliff NT: Management of blow-out fractures of the orbital floor. II. Early
repair of selected injuries. Surv Ophthalmol 35:280, 2991 34. Putterman AM: Management of blow-out fractures of the orbital floor. III A conservative
approach. Surv Ophthalmol 35: 292, 1991 35. Wilkins RB, Havins WE: Current treatment of blow-out fractures. Ophthalmol 89:464, 1982 36. Manson PN, Iliff NT: Management of blow-out fractures of the orbital floor: II. Early
repair of selected injuries. Surv Ophthalmol 35:280, 1991 37. Putterman AM: Management of blow-out fractures of the orbital floor: III. The
conservative approach. Surv Ophthalmol 35:292, 1991 38. Grant MP, Keenum D, Iwamoto M, et al: Orbital Blowout treatment trial: A controlled randomized trial comparing
early surgery versus late surgery for selected orbital fractures. American Association of Plastic Surgeons, 81st Annual Meeting. Seattle, Washington. April 26–29, 2002 39. Bansagi ZC, Meyer DR: Internal orbital fractures in the pediatric age group: Characterization
and management. Ophthalmol 107:829, 2000 40. Sires BS, Stanley RBJr , Levine LM: Oculocardiac reflex caused by orbital floor trapdoor fracture: An indication
for urgent repair (Letter). Arch Ophthalmol 116:955, 1998 41. Berkowitz RA, Putterman AM, Patel DB: Prolapse of the globe into the maxillary sinus after orbital floor fracture. Am J Ophthalmol 91:253, 1981 42. Smit TJ, Koornneef L, Zonneveld FW: A total orbital floor fracture with prolapse of the globe into the maxillary
sinus manifesting as postenucleation socket syndrome (Letter). Am J Ophthalmol 110:569, 1990 43. Yab K, Tajima S, Ohba S: Displacements of the eyeball inorbital blowout fractures. Plast Reconstr Surg 100:1409, 1997 44. Jin HR, Shin SO, Choo MJ, Choi YS: Relationship between the extent of fracture and the degree of enophthalmos
in isolated blowout fractures of the medial orbital wall. J Oral Maxillofac Surg 58:617, 2000 45. Raskin EM, Millman AL, Lubkin V: Prediction of late enophthalmos y volumetric analysis of orbital fractures 14:19, 1998 46. Burm JS, Chung CH, Oh SJ: Pure orbital blowout fractures. Influence of time of repair and fracture
size. Plast Reconstr Surg 103:1839, 1999 47. Biesman BS, Hornblass A, Lisman R, Kazlas M: Diplopia after surgical repair of orbital floor fractures. Ophthal Plast Reconstr Surg 12:9, 1996 48. Flood TR, McManners J, el-Attar A, Moos KF: Randomized prospective study of the influence of steroids in postoperative
eye-opening after exploration of the orbital floor. Br J Oral Maxillofac Surg 37:312–315, 1999 49. Segrest DR, Dortzbach RK: Medial orbital wall fractures: Complications and management. Ophthal Plast Reconstr Surg 5:75, 1989 50. Miller GR, Glaser JS: The retraction syndrome and trauma. Arch Ophthalmol 76:662, 1966 51. Edwards WC, Eidley RW: Blowout fracture of medial orbital wall. Am J Ophthalmol 65:248, 1968 52. Linberg JV: Orbital compartment syndromes following trauma. Adv Ophthal Plast Reconstr Surg 6:51, 1987 53. Linberg JV: Orbital emphysema complicated by acute central retinal artery occlusion: Case
report and treatment. Ann Ophthalmol 14:747, 1982 54. Fleishman JA, Beck RW, Hoffman RO: Orbital emphysema as an ophthalmologic emergency. Ophthalmology 91:1389, 1984 55. Schonder AA, Salz JJ, Magnus WW, Castner DVJr : A conjunctival approach to repair of fracture of medial wall of orbit: Case
report. Ann Ophthalmol 4:297, 1971 56. Rumelt MB, Ernest JT: Isolated blowout fracture of medial orbital wall with medial rectus muscle
entrapment. Am J Ophthalmol 73:451, 1972 57. Shorr N, Baylis HI, Goldberg R: 58. McLachlan DL, Flanagan JC, Shannon GM: Complications of orbital roof fractures. Ophthalmology 89:1274, 1982 59. Wesley RE, Anderson SR, Weiss MR, Smith HP: Management of orbital-cranial trauma. Adv Ophthal Plast Reconstr Surg 7:3, 1988 60. Converse JM, Smith B, Wood-Smith D: Deformities of the midface resulting from malunited orbital and naso-orbital
fractures. Clin Plast Surg 2:107, 1975 61. Manson PN: Dimensional analysis of the facial skeleton: Avoiding complications in
the management of facial fractures by improved organization of treatment
based on CT scans. Probl Plast Reconstr Surg 1:213, 1991 62. Watamull D, Rohrich RJ: Zygoma fracture fixation: A graduated anatomic approach to management based
on recent clinical and biomechanical studies. Probl Plast Reconstr Surg 1:350, 1991 63. Mayer MH, Manson PN: Plate and screw fixation in craniomaxillofacial skeletal fractures. Probl Plast Reconstr Surg 1:290, 1991 64. Ellis DS, Toth BA, Stewart WB: A micro system for rigid bony fixation in orbital surgery. Ophthal Plast Reconstr Surg 7:144, 1991 65. Iliff NT: The ophthalmic implications of the correction of late enophthalmos following
severe midfacial trauma. Trans Am Ophthalmol Soc 89:477–548, 1991 66. Gruss JS, Hurwitz JJ, Nik NA, et al: The pattern and incidence of nasolacrimal injury in naso-orbital-ethmoidal
fractures: The role of delayed assessment and dacryocystorhinostomy. Br J Plast Surg 38:116, 1985 67. Stranc MF: The pattern of lacrimal injuries in nasoethmoid fractures. Br J Plast Surg 12:339, 1970 68. Harris GJ, Fuerste FH: Lacrimal intubation in the primary repair of midfacial fractures. Ophthalmology 94:242, 1987 69. Iliff NT: The management of lacrimal trauma occurring with midfacial fractures. Probl Plast Reconstr Surg 1:420, 1991 70. Khan MD, Kundi N, Mohammed Z, Nazeer A: A 6½ years survey of intraocular and intraorbital foreign bodies
in the North-West frontier province, Pakistan. Br J Ophthalmol 71:716, 1987 71. Koval R, Teller J, Belkin M, et al: The Israeli ocular injuries study: A nationwide collaborative study. Arch Ophthalmol 106:776, 1988 72. Miller CF II, Brodkey JS, Colombi BJ: The danger of intracranial wood. Surg Neurol 7:95, 1977 73. Zinreich SJ, Miller NR, Aguayo JB, et al: Computed tomographic three-dimensional localization and composition
evaluation of intraocular and orbital foreign bodies. Arch Ophthalmol 104:1477, 1986 74. Tate E, Cupples H: Detection of orbital foreign bodies with computed tomography: Current limits. Am J Roentgenol 137:493, 1981 75. Hansen JE, Gudeman SK, Holgate RC, Saunders RA: Penetrating intracranial wood wounds: Clinical limitations of computerized
tomography. J Neurosurg 68:752, 1988 76. Reshef DS, Ossoinig KC, Nerad JA: Diagnosis and intraoperative localization of a deep orbital organic foreign
body. Orbit 6:3, 1987 77. Green BF, Kraft SP, Carter KD, et al: Intraorbital wood: Detection by magnetic resonance imaging. Ophthalmology 97:608, 1990 78. Ewart JH: Large piece of glass imbedded in the orbit for 20 years without causing
symptoms: Removal. Lancet 2:315, 1903 79. Duke-Elder S, MacFaul PA: Foreign bodies in the orbit. In Duke-Elder S (ed): System of Ophthalmology, vol 14. St Louis: CV Mosby, 1972:655–669 80. Higgins C: Foreign body lodged in the orbit for forty-six years: Removal. Lancet 1:82, 1891 81. Cooper WC, Haik BG: Management of orbital foreign bodies. In Della Rocca RC, Nesi FA, Lisman RD (eds): BC Smith Ophthalmic Plastic and Reconstructive Surgery, vol 1. St. Louis: CV Mosby, 1987:523–531 82. Sinovich VA, Gudkova EV: The effect of foreign metal bodies in the orbit on the eyeball coats. Vestn Oftalmol 82:10, 1969 83. Burch PG, Albert DM: Transscleral ocular siderosis. Am J Ophthalmol 84:90, 1977 84. Gerkowicz K, Prost M: Experimental investigations on the penetration into the eyeball of iron
administered intraorbitally. Ophthalmologica 188:239, 1984 85. Gerkowicz K, Prost M, Wawrzyniak M: Experimental ocular siderosis after extrabulbar administration of iron. Br J Ophthalmol 69:149, 1985 86. Horiuchi K, Horiguchi S, Utsunomiya T: Laboratory studies on the lead poisoning in six cases with lead shot lodged
in the tissues. Osaka City Med J 15:7, 1969 87. Dillman RO, Crumb CK, Lidsky MJ: Lead poisoning from a gunshot wound: Report of a case and review of the
literature. Am J Med 66:509, 1979 88. Jacobs NA, Morgan LH: On the management of retained airgun pellets: A survey of 11 orbital cases. Br J Ophthalmol 72:97, 1988 89. Bartkowski SB, Kurek M, Stypulkowska J, et al: Foreign bodies in the orbit: Review of 20 cases. J Maxillofac Surg 12:97, 1984 90. Orcutt JC: Orbital foreign bodies. In Linberg JV (ed): Oculoplastic and Orbital Emergencies. Norwalk, Conn.: Appleton & Lange, 1990:183–197 91. Ferguson EC: Deep, wooden foreign bodies of the orbit: A report of two cases. Trans Am Acad Ophthalmol Otolaryngol 74:778, 1970 92. Wright JE: Surgical exploration of the orbit. Trans Ophthalmol Soc U K 99:238, 1979 93. Simonton JT, Arthurs BP: Penetrating injuries to the orbit. Adv Ophthal Plast Reconstr Surg 7:217, 1988 94. Keeney AH: Orbital foreign body management. Trans New Orleans Acad Ophthalmol 22:14, 1974 95. Norris JL, Cleasby GW: Endoscopic orbital surgery. Am J Ophthalmol 91:249, 1981 96. Perry JD: Transcaruncular approach to the medial orbit and orbital apex. Ophthalmology 107:1459–1463, 2000 |