Skin flaps and musculocutaneous flaps are most often used in lid reconstruction or for filling partial-thickness defects. These usually can supply tissue coverage for the entire anterior lamellae of the lid and can support grafts to the posterior portion of the lid. Alloplastic materials in reconstruction can also be supported. Mucosal flaps with or without tarsus may conversely rebuild the posterior lid and support a free skin graft anteriorly.
In addition to vascular supply, flaps are characterized by their shape. Four types are described: sliding, advancement, rotation, and transposition. A sliding flap is generally random and involves simply undermining the area surrounding a defect to allow primary closure. The skin and subcutaneous tissue is “slid” into position. An advancement flap is a more complicated sliding flap in that the tissue to be “advanced” is incised on three sides to allow greater motility. Closure is primary and generates no secondary defect. An example is the Cutler-Beard flap to reconstruct the upper lid.* The Hughes tarsoconjunctival flap* is another. The rotation flap is similar to an advancement flap in its basic construction in that a three-sided incision and elevation of tissue is performed. However, the defect to be filled is adjacent to the side of the flap rather than at its end, so that the flap is “rotated” into the defect. In doing so, a secondary defect is created opposite the primary. The secondary defect may be closed with a number of techniques, including primary closure if small enough, by developing sliding flaps (further undermining) perhaps with excision of small amounts of additional tissue to produce tension in a different direction, by additional smaller flaps or by a skin graft. Examples include the Tenzel semicircular flap and the Mustardé cheek rotation flap.* The transposition flap moves tissue to a nonadjacent site. Small transposition flaps, like small rotation and advancement flaps, may be random. Larger (i.e., longer) flaps, as in the midforehead,* more often need axial design for proper survival. Examples of transposition flaps are simple Z-plasties, rhomboid flaps, bilobed flaps,* and the tarsoconjunctival flaps described by Hewes and Leone (see Marginal Defects section).
See Figures later in the chapter
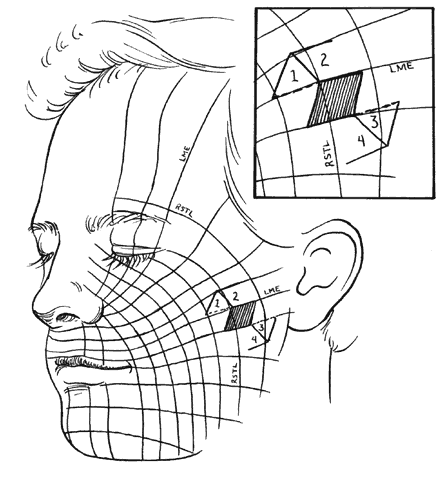
Specific incision placement for these procedures goes beyond the scope of this chapter, but some general comments and concepts are important to include. The concept of the relaxed skin tension lines (RSTL) (Fig. 1) or Langer's lines helps incision placement.5 Wrinkles and skin creases generally develop parallel to these lines as a result of the action of underlying musculature. Incisions ideally should be placed in preexisting creases because they are best hidden in these areas and avoid development of scars that may be more visible. If this is not possible, they should be parallel to the RSTL. The lines of maximum skin extensibility (LME) are perpendicular to the RSTL. The skin is most extensible (i.e., available for sliding, transposing) in the direction of the LME. Vectors of tension should be parallel to the LME. Once the possible donor sites are identified (there are usually several), the site with the most available skin is identified by pinching it up and pushing and pulling the surrounding structures to judge possible distortion caused by closure. For instance, significant lower-lid ectropion might be caused by one donor site and avoided by an alternate. This should be kept in mind when designing flaps and will be expanded further in the discussion of rhomboid flaps.
DEFECTS THAT DO NOT INVOLVE THE LID MARGIN
Of course the most common method of closing a skin defect is simple direct closure, perhaps with just a little undermining. Undermining produces a sliding flap. However, the size of a defect or its proximity to another vital structure, such as lid margin, punctum, or canthus, may make primary closure difficult or may produce an unwanted side effect, such as ectropion or lagophthalmos. In this case, a flap closure may be appropriate. Additionally, skin grafts certainly can be used, especially with difficult-to-close areas, such as the medial canthus. Lid skin, being thin and elastic, often allows direct closure with sliding or simple rotation or advancement flaps. Cheek and brow skin is much thicker and less mobile, requiring more extensive undermining and more frequent transposition of skin.
Z-Plasty
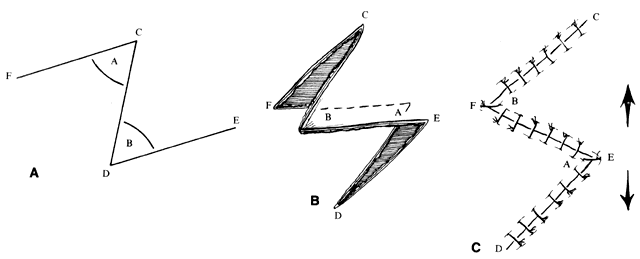
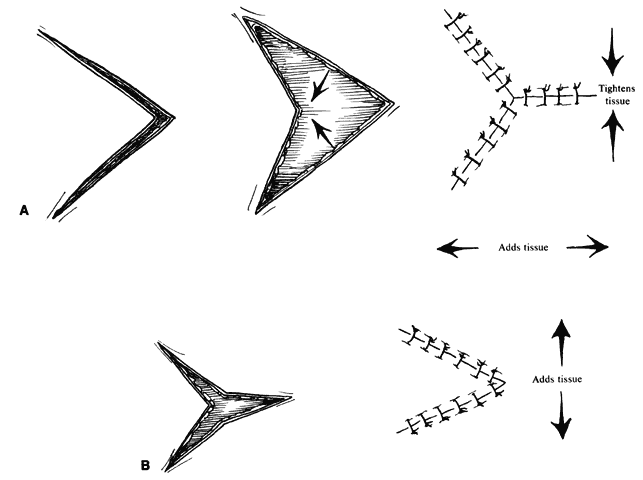
Z-plasty (as well as V-Y and Y-V) is one of the most basic techniques: it may be used in many simple as well as more complicated repairs. This technique (Fig. 2), which employs transposition of two flaps of skin, brings tissue from one direction and adds it to another perpendicular to the first. Z-plasty involves marking and dissecting two triangular flaps, which are transposed. The center of the Z (see Fig. 2, line CD) is aligned on the “short” axis of an area to be lengthened (such as a contracted scar). Each arm of the Z is equal in length and forms angles from 30° to 90°, with angles of up to 60° being the most useful. Larger angles provide the most lengthening but are technically more difficult and are rarely useful. The greater the angle, the greater the percentage gain in length along the central axis; the longer the central member, the greater the actual increase in length.6 A Z-plasty, in addition to being used in other reconstructions, can be used to lengthen a contracted scar, to realign a scar to fall in natural folds (along RSTL), or to make the scar less conspicuous by interrupting its continuity. Long scars can be revised by multiple Z-plasties along their length. Malpositioned structures, such as the brow or canthus, may be repositioned by including them in one of the flaps of a Z-plasty.
V-Y and Y-V Plasty
The Y-V and V-Y flaps (Fig. 3) are sliding flaps that also may be combined with other techniques, such as the five-flap procedure for epicanthal folds, which is discussed later. They may be used to close the donor site of another skin flap or to relieve tension on the lid margin, as from a tight scar. The flap is developed by making a V-shaped incision and recessing the central flap, closing to form a Y, (see Fig. 3A) or reversing the process by making a Y-shaped incision, advancing the flap to close as a V (see Fig. 3B). The Y-V flap is useful in the correction of epicanthal folds.
|
Subcutaneous Pedicle Flaps
The subcutaneous pedicle flap is a flap of skin that is totally divided from surrounding skin but is left attached to the subcutaneous tissues for its blood supply. Adequate mobilization of the pedicle may allow greater mobility of the skin, and this flap usually combines the technique of V-Y closure. It may be used to close a number of defects in a fashion similar to the V-Y-S flap (Fig. 4A and B), described in the paragraph on medial canthal defects. The flap is elevated by incising all sides of a triangle and gently mobilizing the deep tissues. The skin may then be advanced to fill a defect, with closure of the secondary defect directly (Fig. 4C and D). Two opposing flaps may be elevated for larger defects. With adequate design, flaps such as the V-Y glabellar flap and variations of the median forehead flap (see V-Y Glabellar Flap section) can be developed with a subcutaneous pedicle and advanced to fill medial canthal, lower-lid, or cheek/nasolabial defects. This has the advantage of a one-stage procedure with a flap that can be shaped to lie flat without “dog-ears” of skin. The pedicle must be long enough to reach the recipient site without tension or kinking and to provide vascular elements sufficient to sustain the flap.7
Rhomboid Flaps and Variations
The rhomboid flap5 is very useful for reconstructing cheek defects. It can also be used with some medial canthal or brow and temple sites. Because the flap has a precise geometric design, its principles help us to understand the principles of flap design and transfer and to execute other transposition flaps. Because of the rich vascularity of the cheek and relatively little concern of viability, randompattern flaps are the rule. They are raised at the level of the subdermal plexus, leaving a layer of subcutaneous tissue attached to the skin. Transposition skin flaps, such as the rhomboid flap, are designed adjacent to the defect and share all or part of one side of the defect.
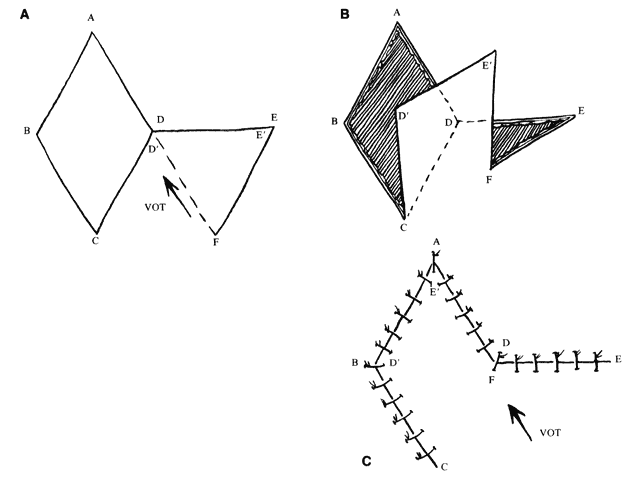
The flap shapes may vary, for instance being oval rather than rectilinear, but can be thought of as having two sides, a distal end, and a base. If spacing and lesion shape allow, and excessive removal of normal skin is avoided, the rhombic shape is marked around the lesion with two sides parallel to the LME. These lines run perpendicular to the RSTL (see Fig. 1). As will be seen, the vector of traction will be roughly parallel to the LME if well constructed (see Fig. 1 inset; Fig. 5), producing the easiest closure. The flap is ideally drawn with all sides and the short diagonal equal (see Fig. 5, line D'F) in length. Essentially this represents two equilateral triangles base to base. The distal end of the flap (see Fig. 5, point E) is a continuation of the short diagonal of the defect (in effect, the base of the triangles). The sides of the flap, the edges of the defect, and the continuation of the short diagonal are all of equal length. There are four possible donor sites for any given rhomboid defect, two on each side of the defect on the extension of the short diagonal. These can be mentally drawn on the defect to decide which is best (see Fig. 1, inset). The vector of tension (VOT) is roughly parallel to the short diagonal of the flap (see Fig. 5A). If the defect is properly positioned with two sides parallel to the LME, the VOT of two of the possible four flaps will be parallel to the LME (see Fig. 1 inset—dotted lines, flaps 1 and 3). The other two possible flaps will have VOT roughly perpendicular or close to parallel to the RSTL, which is an undesirable condition (see Fig. 1 inset, flaps 2 and 4). Either of the preferred flaps can be used, but proximity to other structures will usually make one preferable over the other. The surrounding skin is pushed, pinched, or pulled to assess the possible distortion produced by each flap and the most advantageous one picked.
The near corner of the flap base is adjacent to the defect (see Fig. 5A, point C). The far corner of the flap base is called the pivot point of the flap (see Fig. 5A, point F). It is the width of the flap away from the near corner. The pivot point in a classic transposition flap does not move, but this requires a longer flap for closure. However, with this type of rhomboid flap, the pivot point moves toward the junction of the short diagonals of the defect and the flap (see Fig. 5A, point D). This facilitates closure of the donor defect and eliminates the need for a longer flap. If there is adequate tissue and the flap is well designed, it closes nicely with little tension and the VOT is parallel to the short diagonal of the flap (see Fig. 5A, D'F). However, as is sometimes the case, the pivot point is not mobile enough to close the defect easily. In that case, the junction point of the short diagonals (see Fig. 5A, point D) must move toward the pivot point. For this to occur, a near side of the defect Üol 0Ý(see Fig. 5A, line AD) must elongate, the end of the defect (see Fig. 5A, point A) must move toward the pivot point (which tends to widen the defect), or the far side of the defect (see Fig. 5A, line ABC) must move toward the flap. These movements change the shape of the defect and the flap and will induce a second VOT in the axis of the short diagonal of the flap (see Fig. 5C, D'F) as it is sutured in place. If this is excessive, flap viability is compromised.5 In addition, if the far side of the defect moves, it may distort adjacent structures, which is also undesirable; however, undermining the far side of the defect may allow closure with less tension if the pivot point is poorly mobile.
If the VOT is not ideal, it can be changed somewhat by extending or shortening the far side of the flap. By extending the far side of the flap (see Fig. 5A, line EF), the pivot point is moved more toward the long axis of the defect (see Fig. 5A, line AC). The more parallel the VOT is to the long axis of the defect, the less tendency there is for the defect to widen. However, this also moves the pivot point farther away from the junction of the short diagonals, making it more difficult to close the donor defect.
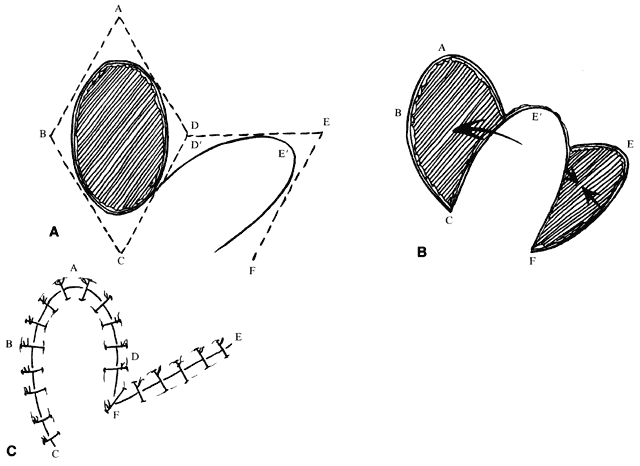
The concepts of the rhomboid flap may also be used with round, oval, and teardrop-shaped defects. For instance, with an oval defect, a rhombus may be drawn around the defect, the flap marked, and then an oval flap designed within the rhomboid to be transposed in a similar fashion (Fig. 6). The principles are the same. In this setting, however, there is somewhat more flexibility in the length of the far side of the flap and its angle with respect to the long axis of the defect. As noted, this allows some modification to the VOT. A longer far side of the flap (see Fig. 6B, line EF) and greater angle between the axis of the flap and the axis of the defect produces a VOT more parallel to the long axis of the defect. (Fig. 6A and B, angle between lines AC and EC, can be changed with oval defects, in contrast to true rhomboids.) As the long axis of the flap rotates, the VOT rotates in the same direction. A VOT more perpendicular to the axis of the defect produces a tendency for the defect to increase in width with closure. A VOT more parallel tends to pull downward on the proximal side of the defect without tending to widen it.5
|
Bilobed Flaps
An additional variation on the rhomboid theme is the bilobed flap (Fig. 7). This flap can be used when it is believed that the flap necessary to close a given defect will leave a donor site too large to close primarily. An additional, smaller (about one-half) flap is elevated adjacent to the donor defect and rotated at the same time, leaving a smaller secondary donor defect, which can be closed primarily. This can be used multiply in some areas of thick, inelastic skin, such as around the nose.
|
V-T Closure
Harvey and Corin8 described a simple closure of skin defects not involving the lid margin or lashes. In the typical case of a tumor overlying the tarsus, the lesion is excised by producing a base-up triangle. An infralash incision is performed medially and laterally, extending the base of the triangle as necessary with undermining to allow rotation of skin flaps into the defect. Closure gives horizontal rather than vertical tightening and produces little vertical scarring forces. Lesions close to the lid margin are effectively removed in cases where a vertical ellipse, an alternative closure, may be difficult.
Other
Larger defects in the anterior lamellae, both superior and inferior, can be closed with larger advancement myocutaneous flaps, as described by Anderson and Edwards.9 In the upper lid, they used classic advancement flaps, which may include portions of the brow if necessary to fill upper nasal brow defects. The flap is dissected widely and extends far temporally to allow sufficient advancement. In the lower lid, the flap has similar features to the Mustardé flap in that dissection can be carried temporally as far as the ear if necessary. In this case, however, only the anterior skin muscle lamellae are moved. Flaps of this type can cover large defects if adequately developed.
MARGINAL DEFECTS
Marginal defects can be traumatic in origin, such as from severe motor vehicle accidents with loss of tissue or from human, dog, or other animal bites. More commonly, perhaps, they are the result of excision of malignant tumors.
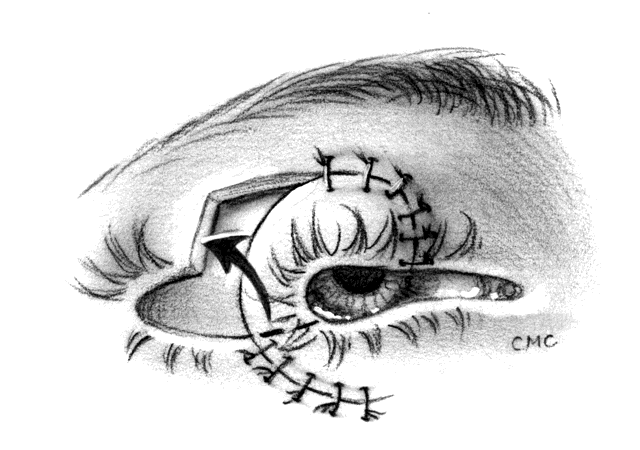
Techniques vary somewhat for upper and lower lids and depending on whether they are nasal or temporal. A combination of two or more may allow closure of a defect in a more advantageous way than a single one, even though the final result may be similar. For instance, a large (greater than 50%) defect in the nasal lower lid too large to be closed by a Tenzel semicircular flap10,11 might be closed by a Hughes-type tarsoconjunctival flap with a skin graft. This requires closing the central visual axis for up to 6 to 8 weeks. This is undesirable, especially if it involves the only seeing eye. An alternative would be to use a rotation flap of the remaining lower lid moved nasally by performing a lateral canthotomy and cantholysis, thus creating a secondary defect laterally. The secondary defect may then be reconstructed with a rotation flap of tarsus and conjunctiva and a skin flap or graft from the upper lid. This produces a somewhat less objectionable lateral tarsorrhaphy, which will not obstruct vision as much and can be opened at a later time. As will be discussed further on in the chapter, Jordan and co-workers12 believe that nearly any defect bordered by remaining tarsus may be closed by their technique, which is a modification of the Tenzel flap. Yet a better approach might be a transposition flap of tarsus and conjunctiva from the upper lid, with a transposition skin flap or graft from the upper lid producing a reconstruction with no tarsorrhaphy.13,14 These techniques will be briefly illustrated, but there are many possible combinations to fit differing lids, locations, and sizes of defect.
In addition to studying the chapter in this text on general lid surgery3 to review the basic principles of techniques such as lid closure, suturing, and needles, we recommend becoming familiar with lateral and medial tarsal strip procedures and their modifications, as described by Anderson15,16 and others.17,18 These procedures are useful in a wide variety of oculoplastic operations, especially in some reconstructions. Direct closure of a lid defect has numerous advantages, including lack of obstruction of visual axis caused by sharing techniques, no need for tarsal substitutes (see grafts and alloplastic materials), no need for multistage procedures, a less complicated and quicker surgery, and good cosmesis. However, many times more complicated procedures are necessary.
Sliding/Rotation Flaps
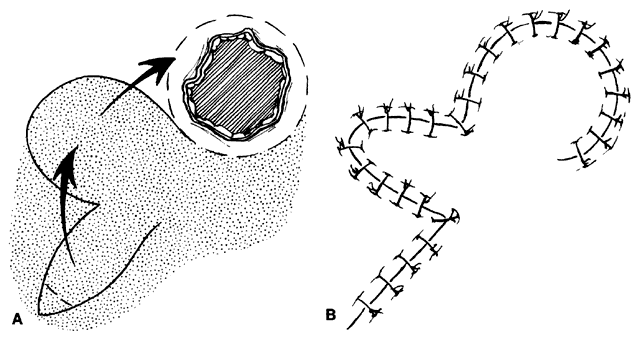
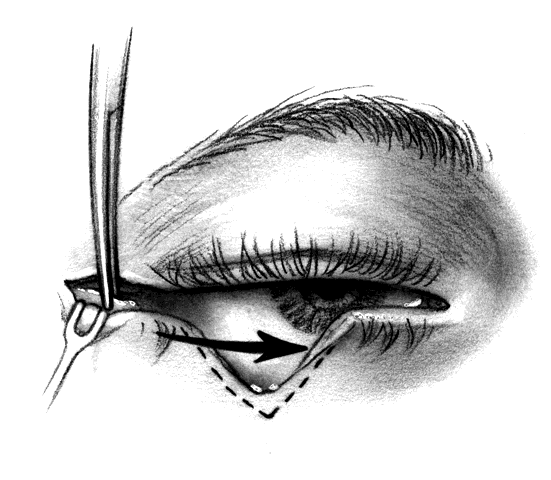
CANTHOTOMY AND CANTHOLYSIS.Lateral canthotomy and cantholysis, which can be applied to either the lower or upper lid, is probably the most useful technique in lid repair when simple primary closure is inadequate. This will allow closure of defects of 25% to 40% or slightly more if the lid is particularly lax. The procedure is simple and is performed with straight, fairly heavy scissors. They are placed horizontally at the lateral canthal angle, and the skin, tendon, and conjunctiva are divided. If fine enough, these same or a smaller pair of scissors are placed on the tendon between the skin and conjunctiva superiorly or inferiorly, and the tendon is divided down to the orbital rim (Fig. 8). The lid can be felt to release and slide nasally when the tendon is completely divided. There are often several strands that fan inferiorly that need to be cut for complete release. If conjunctiva is divided, it can be closed or the open area left to re-epithelialize. The skin is closed laterally at the canthus. Many defects can be closed with this simple technique, which gives excellent cosmetic results.
|
If a canaliculus is nonfunctioning and can be sacrificed, a medial canthotomy and cantholysis can be performed in selected cases.19 This can also be combined with a similar lateral procedure to allow closure of central defects without sharing procedures or grafts.
TENZEL ROTATION FLAP. In the mid to late 1970s, Tenzel10 and later Tenzel and Stewart11 described the semicircular flap to reconstruct the central one half of the lid. The results of earlier operations in this area were not as satisfactory as the results with Tenzel's technique, which has since become popular. Jordan and colleagues12 reported on some modifications of this technique in a medium-sized series. They found that they were able to close defects of 50% to 70% of the central and medial margin and defects of almost any size if medial and lateral stumps of tarsus remained. Their modifications involve making a more vertical incision at the canthus, more extensive undermining laterally, and if necessary, division of the conjunctiva and lower-lid retractors to allow further mobilization of lid remnants. Their extensive lateral dissection has the disadvantage of possible injury to the facial nerve (cranial nerve VII).
The semicircular flap technique as originally described10 involves making a skin and muscle incision at the lateral canthus, which curves superiorly and temporally in a semicircular fashion (Fig. 9). A lateral canthotomy and cantholysis are performed to slide the lateral portion of the lid nasally. Of course, this assumes there is a remnant of tarsus available laterally. If there is not, other procedures may be used, such as a sharing procedure from the upper lid with advancement or transposition flaps or a graft. Closing the primary defect with standard techniques may require removing a piece of conjunctiva inferiorly under the primary defect. Tenzel suggested using this tissue to reline the flap laterally. The semicircular skin flap stretches and moves into the area vacated by the tarsal remnant and forms the anterior lamellae of the lid in that area. With slight undermining and sliding, the semicircular flap temporally can be closed primarily. Small dog-ears (Burows' triangles) of skin may need to be removed to achieve a flat closure.
|
Especially with larger defects, the modification by Jordan and associates,12 wherein they suggested a more vertical incision and inclusion of additional deep muscle by making a beveled incision, would seem to provide some advantage and more lateral support to the new lid. However, the more extensive lateral dissection has the disadvantage of risking injury to the facial nerve. Indeed, as they pointed out, with larger procedures this becomes very similar to the well-known Mustardé rotation flap20 (see next section), although the dissection is more superior (temporal area rather than cheek).
As with the Tenzel10,11 flap, the modification of Jordan and colleagues12 can be used with upper-lid reconstructions as well. The semicircular flap or vertical incision is made inferiorly instead of superiorly, the superior limb of the canthal tendon is divided, the lid remnant is moved nasally, and the skin muscle flap is used to reconstruct the lateral portion of the lid. Careful closure laterally to reattach the new portion of lid to the remaining tendon of the lid is important for support and redevelopment of the canthal angle.
Jordan and co-workers12 also pointed out that their technique ends with apparent excess of tissue at the lid margin in the reconstructed area. This tissue will contract and smooth with time, which may help prevent a lid notch. With large defects, other procedures may be combined with the Tenzel-type flap. McCord and Nunery21 suggested the addition of a periosteal flap elevated from the lateral orbital rim to support the lateral part of the lid. Careful placement of this flap gives good upward support to the lateral lid. A strip about 5 mm in width, with a length appropriate for the defect size, is elevated from the rim and hinged at the orbital rim. It is sutured to the lid remnant nasally and effectively produces a new canthal tendon. The anterior lamella is reformed by the semicircular flap. The inner aspect is lined with elevated conjunctiva. If a large lateral defect is present, an ear cartilage graft (or, for that matter, a nasal chondromucosal, hard palate mucosa, or tarsoconjunctival graft) can be used to reform the inner lamella laterally, with the semicircular flap to reconstruct the anterior lid. Both these modifications can be used with lateral defects that extend to the canthus, where there is no tarsal stump to advance.
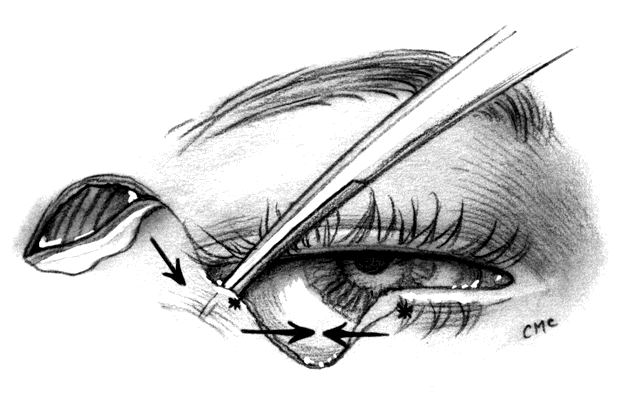
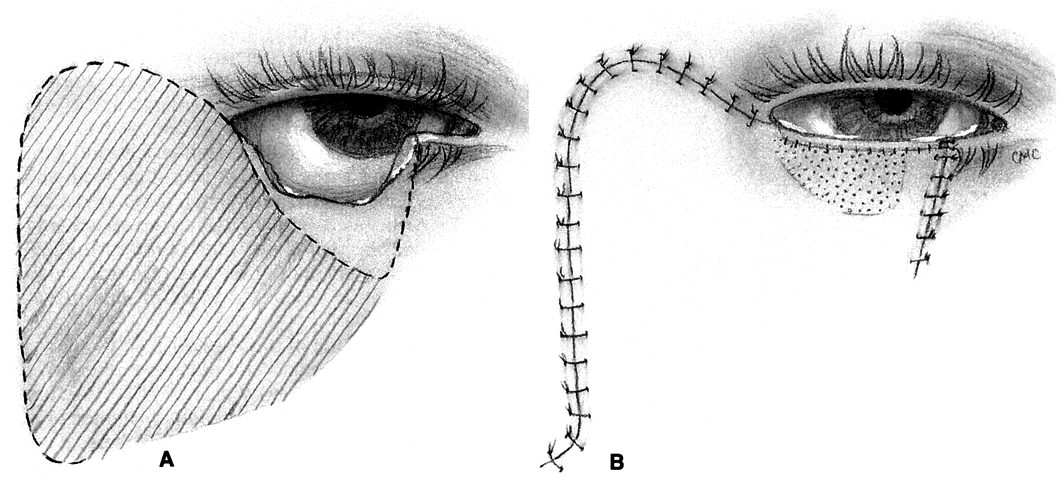
MUSTARDÉ CHEEK ROTATION FLAP. The Mustardé flap20 has the reputation of being a major operation. If necessary, it can be used to close big defects that are difficult to manage by other techniques. Although we perhaps see very large primary tumors less frequently now than in the past, recurrent lesions (especially morphea-type basal cell carcinoma) can be widespread by the time they are detected, resulting in large defects at the time of removal. Large skin grafts require the use of less than optimum donor sites (see Grafts section) or two or more sites. If possible, split-thickness grafts should not be used on the lids or face to avoid contraction. A large flap, with grafts for the posterior lamella, may therefore be the best option and can give satisfactory results when well performed. However, the cheek rotation flap has the inherent disadvantage of producing an inferior vector of traction during healing, which is aggravated by gravitational forces; this makes ectropion or lid retraction with scleral show more likely. Other disadvantages include a large scar and the possible necessity of excising considerable amounts of normal tissue, since in addition to the lid defect from primary tumor excision, a large superiorly based triangle of skin with the nasal edge at the nasolabial fold will also need to be excised. Possible facial nerve damage from the dissection of the full-thickness musculocutaneous flap may also occur.
The flap (Fig. 10) is developed by outlining the area from the lateral canthus extending superiorly and temporally to or above the brow.3,21 This helps to provide superior support to the lateral lid. Many illustrations of this procedure do not emphasize this point. The incision is extended laterally to just in front of the ear and then inferiorly over the jaw. The flap is extensively undermined prior to rotation. The posterior lamella of the lid is reformed by a variety of grafts, again including ear or nasal cartilage, tarsus, or possibly hard palate mucosa. The flap is rotated into position with trimming of the large redundant triangle nasally and of the flap laterally to avoid puckering. If the canthal tendons were removed, careful suturing of the graft and flap to remnants is necessary to provide good support to help prevent ectropion and lid displacement from the globe.
Tarsoconjunctival Flaps
Although many lid defects, even large ones, can be repaired by the relatively easy techniques of canthotomy with cantholysis or semicircular flaps, some defects may be best fixed with a sharing procedure from the opposite lid. Many have been described, including the classic Hughes22 and Cutler-Beard23 operations. Both have been modified, and we will present several examples.
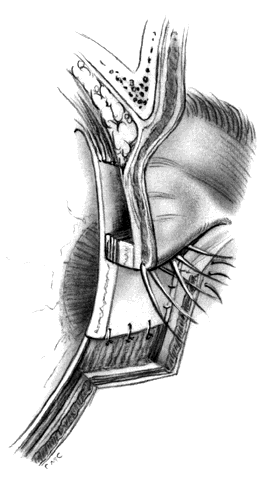
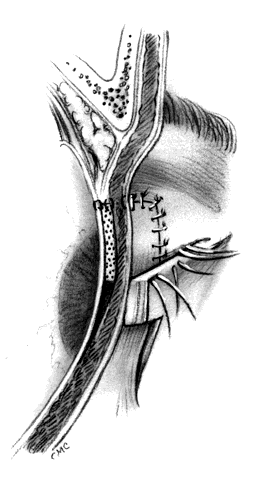
HUGHES PROCEDURE AND MODIFICATIONS. In 1937, Hughes22 presented his first description of the now classic procedure to reconstruct the lower lid and emphasized in a later article the following concept: “When it is necessary to provide new lid structures nothing replaces lid tissue so well as lid tissue itself, both functionally and cosmetically.”24 The original technique involved splitting the upper lid into anterior (skin-muscle) and posterior (tarsoconjunctival) flaps. Because of possible instability or distortion of the lid margin or loss of lashes, this technique has been modified. The generally accepted technique places the incision in the tarsus about 4 mm above the margin, thus leaving the margin intact.
The basic technique for the Hughes procedure has been nicely described by Shore and associates3 and will be outlined only briefly here (Fig. 11). After excision of the lower-lid lesion, the remaining lid segments are drawn toward each other to judge the minimal amount that must be borrowed from the opposite lid. The lid is everted on a retractor or over a plate. In the upper lid, a horizontal incision about 4 mm from the margin is made through tarsus. Vertical incisions are made to the superior tarsal border. The tarsoconjunctival flap is elevated, and the levator muscle of the upper eyelid and Müller's muscle are dissected free. The conjunctiva is left attached and advanced with the tarsus to the lower lid, where the tarsus is sutured to the conjunctiva and lower-lid retractors inferiorly, and medially and laterally to the cut edges of the tarsus remaining in the defect. After thus repairing the posterior lamellae, the anterior can be reconstructed with a skin graft, a transposition flap from the upper lid, an advancement flap from the lower lid inferiorly (increased risk of producing ectropion), or a laterally based advancement flap, depending on the location of the defect and the laxity and availability of skin. The tarsorrhaphy thus created is left in place for 4 to 8 weeks (Hughes suggested 3 months).
After opening the lid, margins and donor site may require some trimming, but will re-epithelialize rapidly. Some redness is expected and will fade in 6 months to a year. Care must be taken not to advance the levator tendon, or else lid retraction will result. This modification has an advantage over the original technique in that it does not violate the upper-lid margin or disturb the lashes. Hughes describes grafting lashes from the brow to the lower lid midway through healing. In many cases, a lower lid without lashes gives an acceptable appearance and avoids the possibility of misdirection with trichiasis, but grafting can be considered if cosmesis requires.
Although this basic technique was described and is best used for lower-lid reconstruction, a “reverse” Hughes procedure has been described for upper-lid reconstruction. Not surprisingly, Hughes reported this himself in his 1945 article24 on lid reconstruction. Several authors, including Leone25 and Mauriello and Antonacci,26 have presented their experience with this modification, which technically is similar to the upper-lid procedure. Jordan and colleagues27 also reported using features of the classic Hughes operation to rebuild the central upper lid in patients in whom complete tarsal excision was not necessary. They found that if at least 3 mm of tarsus remained superiorly, it could be advanced to reform the lid margin with appropriate closure to the remaining lid fragments and could be covered with a graft or advancement flap.
Leone25 used virtually the same procedure, supplemented with a reverse Hughes' flap from the lower lid, the combination filling a large upper defect. As with the standard Hughes procedure and other lid-sharing techniques, the reverse Hughes' flap employs tarsorrhaphy for eye closure, which remains for up to several months (also a disadvantage).
A further variation of the Hughes-type tarsoconjunctival flap to close a lateral upper-lid defect was presented by McCord and Wesley.28(pp80–82) Again, this is a modification of a procedure Hughes previously described in which he used aspects of his procedure to help rebuild the upper lid with smaller (one half or less) medial or lateral defects.24 McCord and Wesley elevated a tarsoconjunctival flap centrally, which in effect is a partial-width Hughes' flap. The flap is used to fill the lateral upper-lid defect by rotating it into the lateral defect where it is sutured to the canthal tendon remnants and the lid margin at the edge of the tarsal defect. The flap is then covered by a skin flap or graft.
We have also used a modification of the Hughes' flap in what amounts to a large Elschnig's tarsorrhaphy laterally to fill lower-lid defects at the canthus produced by directly excising tumors there or by sliding the lid nasally to fill a defect. The upper lid is split, as Hughes originally described, and a triangular flap based at the lateral canthus is rotated inferiorly to the lower-lid defect with appropriate closure of lid margins. The tarsoconjunctival flap is covered with sliding or advancement flaps inferiorly by a transposition from the upper lid, or by a skin graft. This has given very satisfactory results. The tarsorrhaphy is opened in 6 weeks.
An interesting modification of the classic Hughes' flap was presented by Hargiss,29 in which he avoided the occlusion of the visual axis by bringing the flap down on two tubed vascular pedicles constructed of conjunctiva and Müller's muscle. The tarsoconjunctival flap is elevated in the usual fashion without dissecting Müller's muscle from conjunctiva. A horizontal incision is made through conjunctiva about 1.5 mm above the tarsal border centrally and measuring 6 to 8 mm less than the defect width. The extra conjunctiva above the tarsal border folds anteriorly to form a mucosalined margin. Vertical incisions centrally are then made to form two strips of conjunctiva and Müller's muscle, which are sutured to form tubes. The remainder of the operation is completed by standard techniques. After several weeks, the pedicles can be gently pinched to judge the adequacy of neovascularization to the new lower lid before division. Although this has several additional steps, the procedure has the distinct advantage of not occluding the visual axis.
OTHER TARSOCONJUNCTIVAL FLAPS. In addition to the several techniques already described for rebuilding the lateral lower lid, some of which would produce a tarsorrhaphy, the tarsal transposition flaps described by Hewes and co-workers13 and modified by Leone and Van Gemert14 can be useful in producing a nice reconstruction with a one-stage procedure. A tarsoconjunctival flap, based laterally to include the tendon, is elevated from the upper portion of the lateral tarsus. This avoids the lid margin to avoid distortion or loss of lashes, but care should be taken to avoid the fornix as well so as not to damage the lacrimal secretory system. A flap 4-mm wide is adequate and is fashioned so that it just closes the lower defect with slight tension. Leone and Van Gemert14 suggest taking the flap from the center of the tarsus rather than from the upper border, as suggested by Hewes and associates.13 This obviates the need for closure and avoids possible ductule damage. However, Stephenson and Brown30 found that midtarsal donor sites were associated with greater donor lid distortion and did not recommend them. As with other techniques, the anterior surface can be covered with grafts from a variety of sources or the skin and muscle advancement or transposition flaps as suggested by the above authors.
Cutler-Beard and Other Full-Thickness Procedures
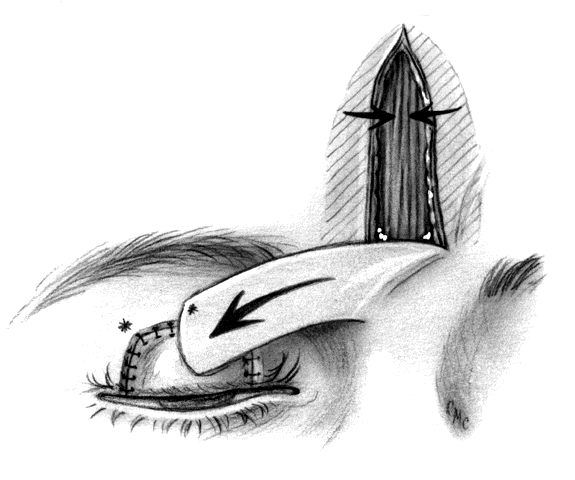
In 1955, Cutler and Beard23 reported a method for repair of partial and total upper-lid defects. As expected, their technique has been modified in an attempt to improve it by avoiding some of its problems. However, the operation is relatively straightforward and gives quite satisfactory results. Basically it involves a full-thickness advancement flap from the lower lid to the upper lid, excluding the margin (Fig. 12). Because the lower tarsus is narrow, it is not included in the flap. The skin, muscle, retractors, and conjunctiva are tunneled under the bridging lid margin and advanced superiorly. The tarsorrhaphy is opened after 2 months. The inferior edge of the margin is freshened and closed to the remains of the flap. Although there is often enough scar tissue or thickening in the flap to give reasonable support to the upper margin and prevent entropion, some believe this lack of tarsal replacement to be inadequate and have modified the procedure by adding sclera,31 cartilage,32 and aorta.33 If completely covered by conjunctiva, an alloplastic material such as polytetrafluoroethylene (Gore-tex, see appendix) might be used.34
|
Disadvantages of the Cutler-Beard procedure include possible necrosis of the bridge flap, a serious problem possibly caused by pressure patching after the procedure, and irregularity of the lid margin, loss of lower-lid lashes, and lid retraction or ectropion. Beyer-Machule35 suggested performing a lid-tightening procedure on the lower lid at the time of reconnection of the bridge and the advancement flap. In addition, there are no lashes in the reconstructed upper lid, a failing of most similar operations. As in other tarsoconjunctival flaps, the need to occlude the visual axis for an extended period is a major drawback.
Anderson and colleagues have presented techniques for full-thickness unipedicle36 and bipedicle37 flaps for lower-lid reconstruction. These techniques present alternatives to the above-mentioned procedures and may be suitable where others have failed or are inappropriate. The authors have reported several advantages: (1) an excellent tissue match in a single-stage reconstruction; (2) lack of occlusion of the visual axis; (3) use of tarsus rather than substitutes in the posterior lamella; (4) functional orbicularis anteriorly; (5) pedicle support to the lower lid; and (6) simultaneous repair of canthal defects. Careful study of their report is recommended. These procedures do, however, have disadvantages: (1) their technical aspects require careful attention to the microvascular anatomy of the lid; and (2) the procedures seem somewhat more difficult than the lower-lid procedures already described.
In cases where lashes in the reconstructed upper lid are desirable, a full-thickness pedicle flap from the lower-lid margin to the upper lid (Fig. 13) can be performed. The procedure that Mustardé described38,39 can be used for near-total upper-lid reconstruction when combined with other techniques to repair the donor lower lid. The operation works best for defects of up to one half of the upper lid and uses up to one quarter of the lower lid. The marginal vessels supply the flap until new vessels develop, supporting the lash follicles. The technique has the disadvantages of being a two-staged procedure and partially occluding the visual axis. Normal lid laxity allows closure of the remaining lower lid directly in most cases, and the 6- to 7-mm flap provides enough length to close the upper lid. In larger defects, a larger lower-lid flap may require lower-lid reconstruction by canthotomy and cantholysis or by a Tenzel flap. The pedicle should be not less than 5 mm to avoid the marginal vessels, although the skin may be incised to within 3 mm of the margin. The underlying orbicularis is left intact. Likewise, the conjunctival surface and partial-thickness tarsus may be incised to 3 mm from the margin. The incisions should not be closed under tension. The hinge of the flap should be centered under the center of the upper-lid defect. Care should be taken to avoid the punctum. With larger defects, the levator muscle of the upper eyelid should be sutured to the flap to avoid ptosis. In 2 to 3 weeks,39 the pedicle can be divided; however, some surgeons recommend up to 4 to 6 weeks.28 Minor revision of the lid margin is performed by excision of small triangles vertically from the pedicle edges to allow the margin to close smoothly.
|
Sutcliffe40 recently presented the use of a total full-thickness advancement flap of the lower to the upper lid. A relaxing incision is made in the fornix to divide the retractors of the lower lid, and the lash follicles and mucosa at the lid margin are excised. The lid is then advanced to attach superiorly to the levator muscle. The entire tarsus is moved to the upper lid, and when the flap is divided after 3 weeks, the lower tarsal border becomes the lid margin and the incision line the upper-eyelid crease. He reported a good functional and cosmetic result, although both eyelids have no lashes. Purported advantages included simplicity of technique and maximum tissue transfer of all lower-eyelid tissues to the upper eyelid.
MEDIAL CANTHAL DEFECTS
Medial canthal defects are usually the result of excision of malignant lesions. If the lesion requires only skin excision, and if the canthal tendons, canaliculi, and deeper structures are not sacrificed, a skin graft will suffice to cover the defect. It heals quickly, has good color and texture match when obtained from eyelid, has little tendency to retract, and conforms nicely to the concavity of the medial canthus. Leaving such defects to heal by secondary intention has also been reported to produce very satisfactory results, although several weeks of healing may be necessary.41 However, larger or deeper defects—especially if sutures, wires, screws, or plastics are necessary to reform the medial lids, tendons, or lacrimal system—require flap coverage with thicker musculocutaneous flaps. The glabellar and midforehead flaps can supply these tissues. Large anterior lamellar defects in the upper lid can also be covered by the midforehead or temporal forehead flap, although these produce a thicker lid skin than a graft or myocutaneous advancement flap from the eyelid itself.9 However, circumstances may dictate such a flap. In addition to these flaps, smaller ones are also described in the following sections. The V-Y-S and rhomboid flaps can also be useful with smaller defects. We will also briefly discuss some methods for repair of epicanthal folds in blepharophimosis.
V-Y-S-Plasty
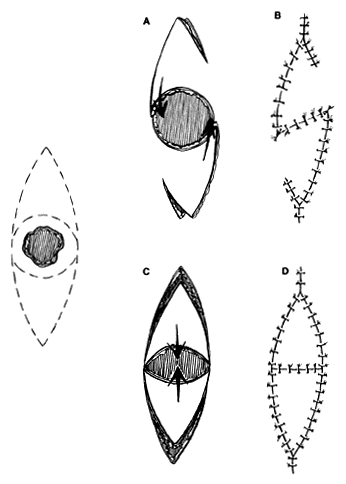
Small, round defects in the medial canthal region can be closed in many ways. Direct closure is possible with small defects, often with undermining. Small dog-ears may need to be excised. However, a straight line closure that crosses the valley of the medial canthal area may bowstring and produce a web. As already noted, skin grafting and healing by secondary intention, or the glabellar flap described later, are options. The V-Y-S-plasty42,43 can be an effective method of closure when direct suturing might distort adjacent structures or produce a web. No undermining in surrounding areas is required or desired. Tissue conservation is maximized. The procedure can be used in other areas of the face as well.
The flaps are designed (see Fig. 4) by outlining the lesion to be excised, with a margin. This is then enclosed by an additional mark, elliptical in shape, that effectively outlines the skin that would be excised and discarded if the defect were to be closed primarily. The long axis is parallel to a natural wrinkle or fold (RSTL) if possible. After excision of the lesion, two marked triangles remain with the defect between. One side of a triangle and the opposite side on the opposing triangle is incised with a short back cut to give an S-shaped configuration. Minimal undermining of the flaps, not the surrounding area, allows them to rotate into the defect for closure. The secondary defects can be closed readily, producing a small V-Y configuration.
V-Y Glabellar Flap
Deep defects in the medial canthus require coverage. Care must be taken in cases of tumor excision to ensure complete removal with frozen-section control. Residual tumor could otherwise be buried, allowing orbital extension. The glabellar V-Y flap (Fig. 14) is taken from the bridge of the nose and glabellar region, occasionally with extension somewhat superiorly to include some skin of the forehead. Color and texture match is reasonably good, and if the defect is deep, the thickness of the skin is not a great disadvantage. Blood supply is good, so a relatively small pedicle will safely support a long flap. Some narrowing of the interbrow distance may be noted but is more a problem with the larger median forehead flap. The flap is raised and rotated/advanced using the V-Y advancement principle (see Fig. 3). The flap can be used to cover defects of up to 15 mm in the medial canthus or larger if combined with other flaps. The height of the flap is about three times its width at its widest inferiorly and is based on the opposite side of the nose. After sliding the flap down, the forehead is closed in an inverted Y. The skin is undermined and usually closes, leaving scars that tend to hide well. The operation has the advantage of being one-staged.
|
Median Forehead Flap
The median forehead flap is used infrequently because other techniques for upper- or lower-lid reconstruction often give better results. Occasionally, however, very large defects of the upper or lower lid and cheek may arise. Circumstances such as loss of both the upper and lower lids due to trauma, or large tumor removal that renders sharing tissue impossible, are rare but not unheard of, and may necessitate its use.
The flap28,44,44a is elevated from the central forehead and is based at the root of the nose (Fig. 15). There is a good blood supply in this area, which makes it possible to create a long, relatively narrow flap. The flap can extend to the hair line if necessary and up to about 2.5 cm in width, slightly more if the forehead is especially loose. The flap is raised in the connective tissue overlying the galea aponeurotica. An estimate of the width of the flap available can be made by making one of the vertical incisions, undermining widely, and overlapping the forehead skin to judge how large a defect can be closed. The superior end of the flap should be tapered to allow smooth closure at the top. Rotating this type of flap will produce a significant dog-ear, which will need to be revised during a second procedure 2 to 3 weeks later. Longer delay may result in the formation of a significant amount of granulation tissue or scarring, or may even result in the pedicle's becoming tubed. However, if this flap is used for total reconstruction of a lid and is lined with a mucous membrane or mucosal-chondral graft, more time must be given for ingrowth of vessels. McCord and Wesley28 recommend 8 weeks. Excising the granulation or scar tissue must be done carefully to avoid damage to the vessels of the flap. The unused portion of the flap may be returned to the forehead after appropriate trimming, thus lessening the narrowing of the interbrow distance.
|
Epicanthal Folds
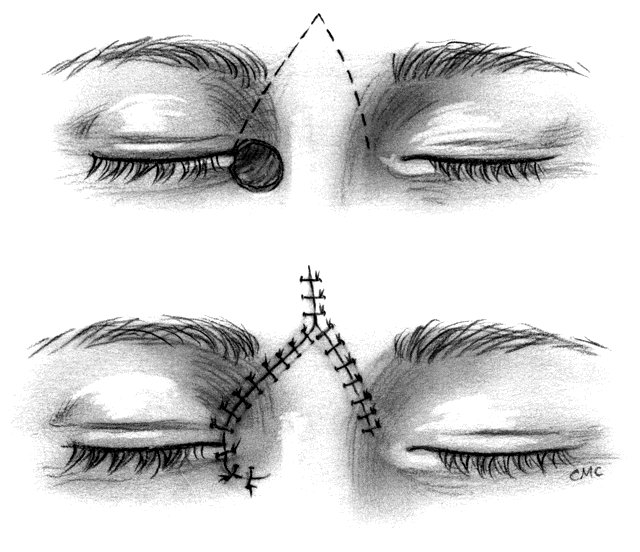
Epicanthal folds occur frequently at birth, but most are minor, produce no significant cosmetic blemish, and diminish with time. Occasionally they are significant and require surgical correction, especially when associated with ptosis in the blepharophimosis-ptosis syndrome. Descriptions of the varieties of folds45 and of the blepharophimosis-ptosis syndrome46 are available elsewhere. Many procedures for the repair of epicanthal folds have been described, including varieties of direct excision, Y-V-plasties, excision of skin from the nose, and a wide variety of flap techniques. All have their disadvantages, including lack of long-lasting effect, scarring, technical difficulty, and suboptimal cosmetic result. Mustardé47 developed a flap technique that improved upon prior procedures and reduced some of the above-mentioned problems. We have used Mustardé's procedure with success but agree with Anderson and Nowinski48 that the measurement of incision lines and angles makes it somewhat difficult. For that reason, we suggest their five-flap technique for this repair in instances where simpler procedures are unsuccessful. The operation combines fairly straightforward double Z-plasty with a Y-V-plasty, thus producing a five-flap operation. It superficially appears similar to Mustardé's procedure, but does not require measurement of lines or angles. Their description also suggests that, after excising excess deep muscle, fat, and fibrous tissue and shortening or repositioning the medial canthal tendon, the flaps fit together more neatly and with less trimming than with Mustardé's procedure.
The basic procedure involves marking the canthus at the medial extent and at a point of the intended new canthal angle. This is approximately one half the distance from the pupil to the center of the nasal bridge. Paramarginal lines superiorly and inferiorly from the canthal angle form the Y. A vertical line through the angle of the Y on the apex of the fold is drawn, and finally back-cut lines equal in length to the paramarginal lines and roughly parallel to them are drawn, thus completing the Z. The flaps are elevated, excess tissue excised, and the Y-V flap advanced with a heavy suture from the canthus to the posterior insertion of the tendon or periosteum. Once the canthus is in position, the flaps are transposed, the apices are closed, and the skin is closed as desired.