
|
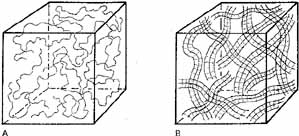
| Fig. 2 A. Sodium hyaluronate molecules in low concentration at rest (zero shear). In solution, the NaHa chain unfolds on itself and forms a long, loose, randomly arranged coil. B. As the concentration of these large NaHa molecules is increased, the individual molecular coils start to overlap and become compressed. This crowding of the chains increases the chances for various noncovalent chain–chain interactions. This, in turn, increases the viscosity of the solution; it also increases the elasticity of the solution. |