The first consideration in providing anesthesia for cataract surgery or any other purpose is understanding the goals of the block. Which parts of the eye does one intend to leave without sensation? Which muscles does one wish to paralyze? Once these questions are addressed, the next task is to understand the cranial neuroanatomy.
The details of trigeminal, oculomotor, and orbital anatomy are beyond the scope of this chapter. The reader is referred to Ocular Anesthesia (WB Saunders, 1997) and Atlas of Clinical and Surgical Orbital Anatomy (WB Saunders, 1994). A brief summary follows.
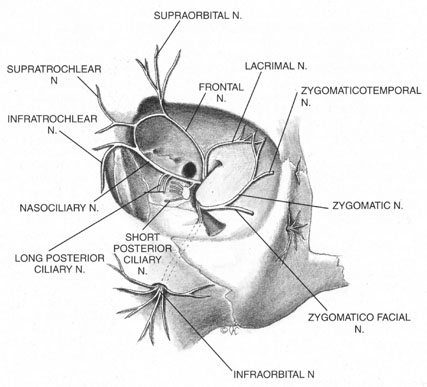
The trigeminal nerve carries the sensory innervation of the eye and adnexa in three divisions: ophthalmic, maxillary, and mandibular. Except for a portion of the sensory input from the lower lid that is carried by the maxillary division, the sensory fibers of the eye and adnexa are found in the ophthalmic division. This division in turn has three components: frontal, lacrimal, and nasociliary, as shown in Figure 1. The frontal nerve usually branches into two more divisions: the supraorbital, which carries sensation from the conjunctiva and skin of the central two-thirds of the upperlid; and the supratrochlear, which carries sensory fibers from the medial third of the upper lid. The lacrimal nerve carries sensory input from the skin and conjunctiva of the lateral aspect of the upper lid.47 The nasociliary nerve carries sensory fibers from the cornea, iris, ciliary body, perilimbal bulbar conjunctiva, and optic nerve sheath; these fibers proceed through its long ciliary branches and sensory root to the ciliary ganglion. The infratrochlear branch of the nasociliary nerve carries sensory input from the medial canthus, medial portion of lower lid skin and conjunctiva, caruncle, lacrimal sac, and canaliculi.
Once it is known which branches are responsible for carrying sensory input from which structures, an approach can be planned that has a reasonable chance of blocking the targeted area. Because, for example, the nasociliary nerve carries fibers that pass through the intraconal space, a standard intraconal retrobulbar block may provide excellent intraocular and partial surface anesthesia. It could not be expected, however, to effectively block the conjunctiva of the upper or lower lids or the lateral aspect of the globe. Because the frontal and lacrimal branches enter the orbit through the superior fissure, above the annulus of Zinn, and the maxillary division enters the orbit through the infraorbital foramen, below the annulus, an intraconal approach probably would not effectively block the structures these branches innervate. If an intraconal retrobulbar block is the only one administered before surgery, patients can be expected to feel irrigating solutions being dropped on the conjunctiva, away from the limbus; they also will be aware of the lid speculum and any manipulation of the lateral surface of the globe. They will probably attempt to close the eye in response to these stimuli. This is the basis for the traditional facial–retrobulbar block combination.
As has been mentioned, numerous facial blocks have been devised to prevent patients from squeezing the eye shut during surgery. None of these blocks keeps patients from wanting to close the eye—only from succeeding. Because the facial block also involves added discomfort, many anesthesiologists and surgeons provide intravenous sedation along with it. Thus, limiting the initial block to the intraconal retrobulbar space also limits its potential benefits, requiring two supplemental procedures. Anatomy determines effect.
The anterior blocks—peribulbar, parabulbar/sub-Tenon's, and topical—reduce or eliminate the need for a separate facial block by providing better surface anesthesia than the retrobulbar block. Although the first two also provide intraocular anesthesia through their effect on the nasociliary branch, topical blocks provide only surface anesthesia, so that intraocular sensations, such as stretching of the zonules during filling of the anterior chamber, may be felt throughout surgery. Some authors advocate the addition of a subconjunctival injection to add more anesthetic effect to a topical block.48 Recently, intraocular injections of local anesthetics have been popularized as an adjunct to topical anesthesia. This technique is discussed later in this chapter.
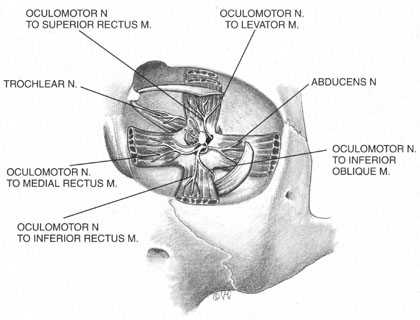
The motor supply of the superior, medial, and inferior rectus, the inferior oblique, and the levator palpebrae superioris is carried by the oculomotor nerve (Fig. 2). It also carries proprioceptive input from these muscles and parasympathetic fibers to the ciliary ganglion. As the oculomotor nerve enters the orbit through the superior orbital fissure, it splits into two divisions, superior and inferior. The superior division is smaller; it courses forward in the superolateral portion of the intraconal space, and turns medially toward the lateral aspect of the superior rectus muscle, where it divides into a network of small branches.49 The innervation of all extraocular muscles is multifocal, with nerve fibers extending distally and proximally between the muscle fibers, before ending at myoneural junctions.47 Some branches innervate the superior rectus, and others pass through it to enter the levator muscle through its inferior surface.
|
The inferior division of the oculomotor nerve splits into at least three trunks within the intraconal space, and these in turn divide into eight to ten branches as they course forward, lateral to the optic nerve. The medial rectus is innervated by branches that run from beneath the optic nerve into the muscle, beginning at its posterior third. The inferior rectus muscle is similarly penetrated at its posterior conal surface by branches of the inferior division of the oculomotor nerve. The inferior oblique muscle is innervated by a branch that initially contains parasympathetic fibers; these fibers originate in the Edinger-Westphal nucleus and enter the ciliary ganglion inferolateral to the optic nerve. The remainder of this branch then breaks up into smaller fascicles, which penetrate the inferior oblique at its posterolateral aspect.47
The trochlear nerve supplies motor fibers to the superior oblique muscle. It enters the orbit through the superior oblique fissure above the annulus of Zinn, along with the frontal and lacrimal branches of the ophthalmic division of the trigeminal nerve. It crosses the superior rectus origin above the levator and enters the superolateral surface of the superior oblique muscle.47
The abducens nerve enters the orbit through the superior orbital fissure, along with the oculomotor nerve. They are sometimes divided by a dense septum connecting the superior rectus origin to the superior rectus sheath.47 The abducens nerve enters the lateral rectus sheath just anterior to the annulus of Zinn, and first enters the lateral rectus muscle at the medial aspect of the junction of its posterior and medial thirds.47,50
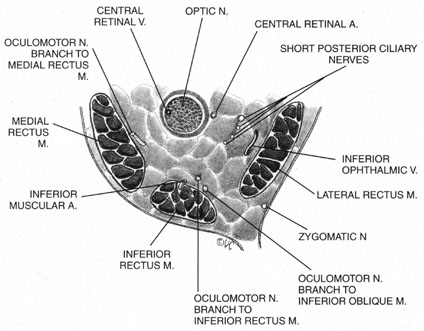
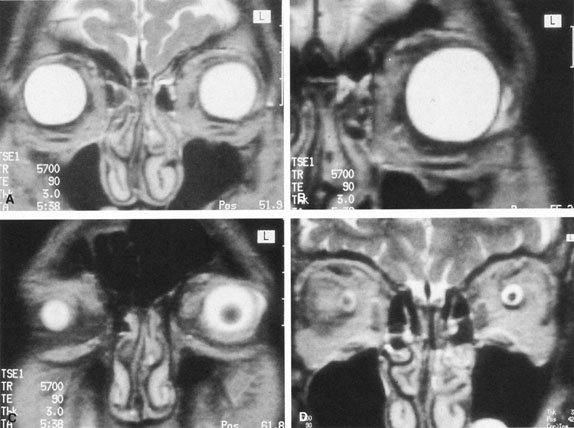
The ciliary ganglion is an irregular structure, measuring 1 mm by 2 mm, that lies just temporal to the optic nerve (Figs. 3 and 4), 7 to 10 mm from the orbital apex.47,51 In it the presynaptic parasympathetic fibers from the Edinger-Westphal nucleus synapse with the postsynaptic fibers that form the short ciliary nerves. Most of these fibers innervate the ciliary muscle, and the remaining 3% to 5% supply the iris sphincter. The ganglion also contains sensory branches of the nasociliary nerve and sympathetic fibers en route to the choroidal vasculature.
|
When attempting to provide akinesia, it should be kept in mind that the motor nerves enter the rectus muscles at the junction of their posterior and medial thirds, or more anteriorly.52 It is also important to remember that these fibers run both distally and proximally between the muscle fibers before they end at the myoneural junctions. A motor block may therefore be achieved at many points along their path. The oculomotor divisions may be blocked in the posterior orbit before their insertion into the rectus muscles, but it is necessary to keep in mind the proximity of the optic nerve, the ophthalmic vein, and the anterior muscular branches to the oculomotor branches in this region (see Fig. 3). Because the nerve and artery supplying the inferior oblique muscle insert more anteriorly, they are more vulnerable to needle trauma from peribulbar or retrobulbar blocks delivered along the floor of the orbit.48 The superior oblique muscle is innervated and receives its blood supply in the posterior orbit. Because it is relatively immobile in the superotemporal orbit, it is possible to injure this muscle with blocks in this area.
Blocks delivered in the anterior or middle orbit depend on diffusion of the anesthetic agent into either the posterior orbit, to the origin of the nerve branches, or into the muscles themselves at the point where distal branches insert into myoneural junctions. The first process is dependent on the anatomy of the connective tissue planes that subdivide the orbit into compartments (see Fig. 4). This architecture varies among patients. The classic teaching of a single intermuscular septum that connects the rectus muscles and divides the orbit into an intraconal and an extraconal space is overly simplified. Histologic examination of the orbit reveals an arrangement of roughly parallel and partially broken septa of various thicknesses, with and without fenestrations.48 This anatomic variability, therefore, accounts for the variability in akinesia seen with orbital blocks.
Atkinson16 discussed supplementing incomplete akinesia by injecting 0.5 to 1.0 mL of anesthetic solution 3 cm back along a rectus muscle, if it was still active after retrobulbar block. He grasped a horizontal rectus muscle with forceps and rotated the eye away from the needle, or placed a muscle hook under the eyelid and separated it from a vertical rectus muscle before injection.16 Because the rectus muscles measure 40 to 42 mm in length without their tendons, which in turn may vary from 3.7 mm for the medial rectus to 8.8 mm for the lateral, Atkinson's injection was administered 14 to 21 mm anterior to the origin of the muscle. This would place it anterior to the insertion of the oculomotor branch innervating the muscle. Because akinesia can be achieved with this technique, the anesthetic must be diffused within the muscle, blocking distal branches of the oculomotor divisions at their most distal myoneural junctions. However, these injections may produce myotoxicity with prolonged muscle paresis.53,54
Because permanent extraocular muscle damage is more a product of intramuscular or intraneural injection than of the concentration of anesthetic injected,48,55 a less traumatic delivery of anesthetic can be used around the extraocular muscles to achieve akinesia without risking either myotoxicity or needle trauma to the adjacent optic nerve and orbital vessels. Using the sub-Tenon's space for this delivery allows for such atraumatic akinesia.