SPECIAL MEASUREMENTS OF VISION
Preoperative measurement of vision is meant to determine the patient's current state of visual function. Snellen acuity is routinely tested on all patients as part of their preoperative evaluation. This usually is performed by having the patient read a standardized chart in a darkened room. Although the Snellen acuity scale is the most ubiquitous measure of vision, it measures only one tiny aspect of visual function. Many patients may be profoundly functionally impaired by their degree of visual disability, yet may test surprisingly well measuring Snellen acuity in a darkened room. In these cases, it is incumbent on the ophthalmologist to seek to better understand and document the patient's problems by performing additional testing.
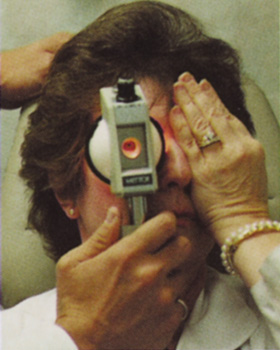
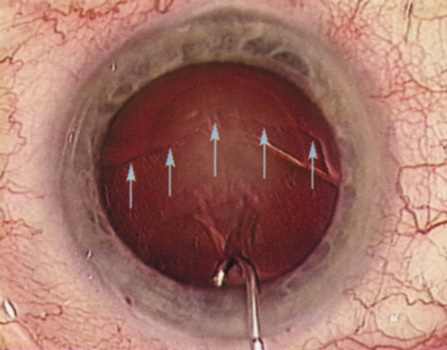
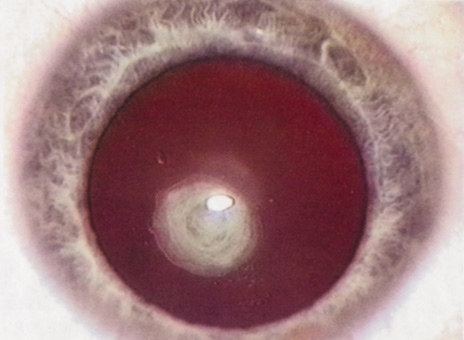
Many patients are most bothered by cataract-induced glare. For these patients, acuity testing under glare situations is indicated. There are several methods to assess visual acuity reduction by glare. The choice of method is often best dictated by the patient's history. If a patient complains of glare problems in the supermarket, or other uniformly illuminated environment, the brightness acuity test can be performed (Mentor Ophthalmics). For this test, the specially illuminated handpiece is held in front of the tested eye using best spectacle correction (Fig. 1). The Snellen acuity is rechecked and can be recorded on each of three light settings.
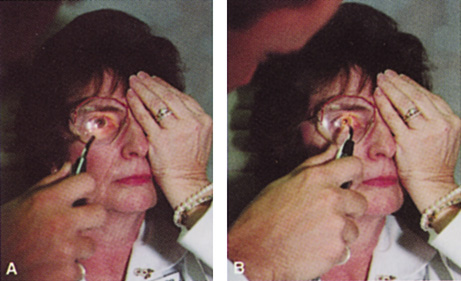
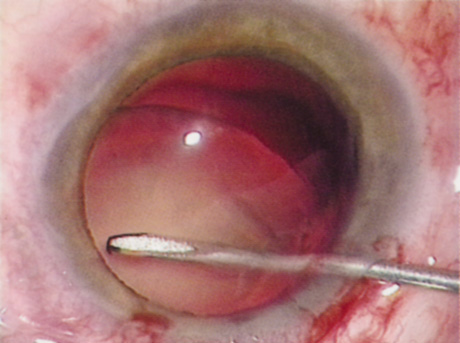
Patients who complain of glare from point sources of light, such as oncoming headlights or bright sunshine, may be best evaluated by a different form of glare testing. To simulate the environment of the patients' symptoms, Snellen acuity is measured while directing a point source of light obliquely toward the eye outside their best spectacle correction or outside of a phoropter dialed in with their best manifest refraction (Fig. 2).
Still other patients' problems may center on difficulty with reading, seeing street signs, or distinguishing fine patterns. In these individuals, the complaints are related more to contrast; therefore, contrast testing is most appropriate. There are a number of ways to assess the effect of contrast on vision. Regan's sine wave gradients have been used frequently for research purposes and are available in some settings. Various commercial devices are now available to measure visual acuity in different contrast settings and each has its relative merits and detractions. The authors have found the Baylor Visual Acuity Tester (BVAT) monitor (Mentor Ophthalmics) testing of contrast to correlate well with patients' complaints and its simplicity is appealing to both patients and technical staff.
In rare instances, patients' complaints may be primarily related to distinguishing colors. Although patients frequently remark about their dramatic improvement in color perception after cataract surgery, there are no convenient methods to document diminished color perception preoperatively. This underscores the importance of correlating patients' complaints with the biomicroscopic examination and the degree of nuclear color change.
PROGNOSTIC TESTS
Physicians often order special tests to help determine a patient's visual potential. Some of these tests are acuity specific. These can be particularly helpful in guiding patients who may have comorbid ocular conditions. Some devices have been designed to project a Snellen chart through the clearest area of the cataractous lens to assess retinal acuity potential such as the potential acuity meter. Studies also have shown a good predictive value by checking vision with a brightly illuminated near card.53 Of course, this can be performed with no additional office equipment. Various other commercial devices, including interferometry and various different pinhole and illumination device combinations, are available.
These approaches are not possible for patients with mature cataracts. Some more general, nonspecific prognostic tests can be performed. If a patient is able to identify the colors of projected lights, this usually indicates that some cone-mediated macular function is present.
Blue field endoscopy also may indicate some macular function. This test is performed by projecting a blue light into the eye. The patient may report seeing small round specks moving around in the vision. These specks correspond to white blood cells passing through the perifoveal capillaries.
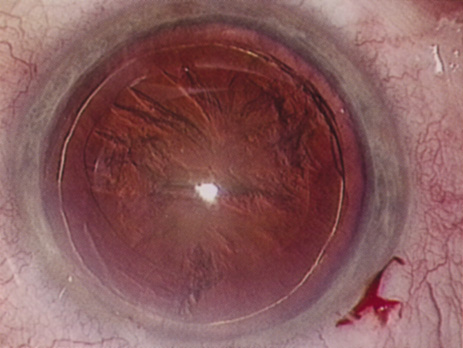
The Purkinje phenomenon is tested easily by rapidly wiggling a transilluminator directed toward the globe through the lower lid in a darkened room. If the patient reports a pattern of crooked lines or branches, then he or she is seeing the shadows cast by the retinal blood vessels, indicating that the posterior pole is attached and functioning to at least some degree. Although positive results from the test are encouraging, some patients may still have limited vision after surgery; similarly, some rare patients may test negatively on all these tests and still recover good vision.
Diagnostic Studies
Several diagnostic studies provide information that supplements the historical and clinical data obtained by the surgeon. This information enables proper preoperative patient consultation and surgical planning. This section outlines many preoperative tests used for cataract patients.
A-SCAN BIOMETRY.
Accurate axial length measurement is critical to determine the correct power of the implant lens for the desired refractive result. A-scan biometry is imperative in any patient undergoing cataract surgery. Both contact (applanation) and immersion varieties of A-scan ultrasound units are commercially available. With applanation biometry, a hand-held or slit-lamp mounted probe is gently touched to the corneal surface along the visual axis. Contact A-scans are user dependent and sometimes the authors adjust the surgeon-specific IOL A-constant depending on which ultrasonographer has performed the scan. Nonetheless, outstanding refractive outcomes have been achieved, and the authors have been satisfied with the contact applanation technique.
With an immersion probe, a water bath around the eye acts as the medium to conduct sound waves. Although there is no direct contact of the probe with the globe, the water and water bath must, of course, remain in contact with the ocular surface and periorbita. Immersion scans may reduce interobserver variations but are less comfortable and less convenient for patients.
A-scan biometry is particularly challenging in eyes containing an oil fill. In this instance laser biometry is still able to achieve excellent measures.
LASER PARTIAL COHERENCE OPTICAL BIOMETRY.
Although ultrasound requires continuous contact with media that conduct sound waves, laser light passes easily through any clear media, including air, making this a truly noncontact or “no touch” test. Furthermore, the speed of light is not appreciably different in the clear media of the eye and thus excellent, reliable measures can be achieved in eyes containing intraocular lenses, regardless of type and eyes with oil fills within the vitreous cavity. Although some calculation adjustments can be made depending on the pseudophakic status, the differences among implant material are not appreciable different from a practical clinical perspective. Currently, the only commercially available laser biometry device is the IOLMaster (Zeiss). The measurements obtained by the IOLMaster device are extremely reliable, reproducible, and seem to be relatively technician- and observer-independent.54,55This device also can measure keratometry, optical anterior chamber depth measurements, and “white-to-white” measurements in an automated fashion. Because it relies on the passage of laser light through the ocular media, this instrument is unable to obtain measurements in cases where the media prevent laser light passage; for example, white cataracts, axial posterior subcapsular cataracts, or corneal scarring.
B-SCAN ULTRASOUND.
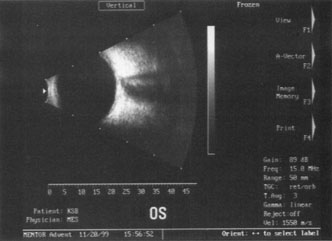
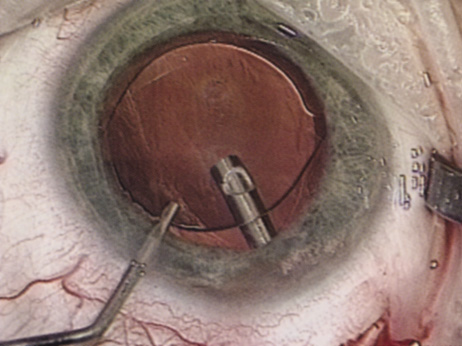
A mature cataract precludes visualization of the fundus. A B-scan ultrasonographic examination provides a real-time, two-dimensional (2D), cross-sectional image of the globe along the marked axis of the probe (Fig. 3). Cataracts are more common in patients with chronic retinal detachment, prior trauma, or intraocular tumors; therefore, a B-scan study is helpful in excluding structural posterior segment pathology before surgery on a mature cataract. Although a negative result to B-scan evaluation is reassuring, the surgeon should remember that it does not predict postoperative visual outcome. The B-scan can be thought of as a picture of Cincinnati from an airplane; the office buildings may all be standing, but you cannot tell whether the people in them are working.
|
ENDOTHELIAL CELL COUNT.
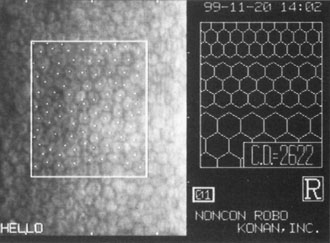
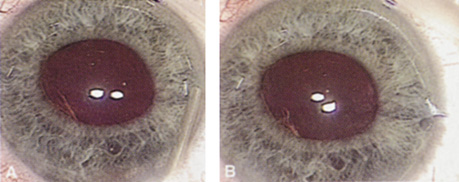
Although slit-lamp examination can give the ophthalmologist an excellent estimate of endothelial health, sometimes a formal assessment of the corneal endothelial cell density is helpful (Fig. 4). This information is most likely to be helpful in advising patients who may be at greater risk of postoperative corneal decompensation. Specifically, patients with cornea guttata, previous ocular surgery, history of blunt ocular injury,56 exfoliation syndrome,57 iridocorneal-endothelial syndromes,58 or a history of glaucoma59 are known to have reduced endothelial cell counts. Patients with a history of acute angle closure are at particular risk because each episode of elevated intraocular pressure can damage endothelial cells.60
There are qualitative and quantitative methods for endothelial cell evaluation. Cell density can be measured directly with an endothelial cell camera. The surgeon also should view the photograph and qualitatively estimate the regularity of the endothelial cell mosaic. Some instruments calculate a coefficient of variability and percent of hexagonal cells.
When an endothelial cell camera is not available, qualitative assessment of count and cell morphology can be accomplished at the slit-lamp using a technique called specular reflection.61 The ophthalmologist focuses a narrow parallelepiped on the corneal epithelium, directing the beam at the periapical cornea from a 45-degree angle. The slit beam is moved slowly from side to side until the bright corneal reflex strikes the examiner's view from the epithelial surface reflection (first Purkinje-Sanson image). On high magnification, the examiner should focus on the endothelial surface just next to the bright reflex. The image of the endothelial mosaic will come into view. The surgeon can make a qualitative assessment of the cell density and degree of regularity. With practice, these estimates can be surprisingly accurate.
The implications of a reduced endothelial cell count are primarily prognostic and can provide the surgeon with more information to help counsel the patient about the risk of corneal decompensation with cataract surgery. Gentle phacoemulsification without triple procedure is recommended when cornea is clear and compact, given that a significant number of patients may be able to avoid a corneal transplant despite uncountable cell densities. However, these patients should be advised that they may be at an increased risk of requiring a corneal transplant.
PACHYMETRY.
Ultrasonic pachymetry measures central corneal thickness. When endothelial function is tenuous, corneal thickness begins to increase gradually, indicating subclinical stromal edema. Similar to endothelial cell count measurements, pachymetry is primarily of prognostic value.
Surgical planning also may differ for the patient with a compromised endothelium. In this case, use a more highly dispersive viscoelastic and minimal amounts of infusional fluids during surgery. When the degree of compromise is severe, additional dispersive viscoelastic material can be added periodically during the phacoemulsification. Because corneal transplant is a distinct possibility, such patients are not good candidates for a multifocal implant.
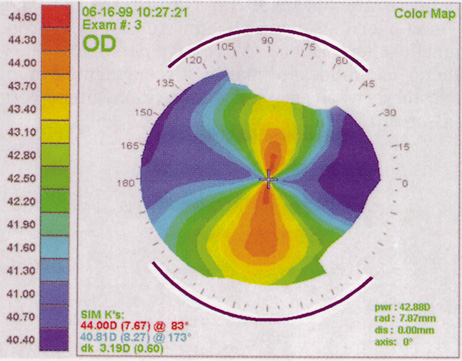
CORNEAL TOPOGRAPHY.
Computed corneal topography may be helpful from both a diagnostic perspective and as an aid to preoperative surgical planning. The diagnostic value of corneal topography is most obvious when keratoconus or irregular astigmatism is suspected. In cases with irregular astigmatism, superficial keratectomy or excimer phototherapeutic keratectomy may be indicated before cataract surgery, particularly when the relevant pathology precludes accurate keratometry.
In some cases, corneal topography is an invaluable aid in surgical planning as well. In patients with high astigmatism, the topography can indicate the best meridian for placement of limbal relaxing incisions (Fig. 5). The topography also may be used to confirm the keratometric measurements.
|
ULTRASOUND BIOMICROSCOPY.
The ultrasound biomicroscope is a specialized, high-resolution form of 2D ultrasound, similar to the B-scan discussed in the preceding. An ultrasound biomicroscope study may be helpful to the cataract surgeon in cases of mature cataract when retroiridial, anterior segment pathology is suspected. Currently, the ultrasound biomicroscope instrument is expensive and not widely available, and has uncommon indications.