INDICATIONS
Despite major advancements in cataract surgery, complete removal of all lens epithelial cells at the time of surgery is currently not possible. The posterior lens capsule and a portion of the anterior capsule remain in place to support a posterior chamber lens during modern extracapsular cataract extraction techniques. Posterior capsular opacification occurs when retained lens epithelial cells from the equator of the anterior capsule proliferate, undergo metaplasia, and then migrate across the posterior capsule. Posterior capsular opacification occurs in two forms: Elschnig's pearls and fibrotic changes from residual cells that undergo metaplastic transformation into myofibroblasts.1,10 In the past, visually significant PCO requiring YAG capsulotomy occurred in 57% of eyes at 3 years after cataract surgery.5 However, with current techniques, this figure has been significantly reduced.
The visual symptoms produced by the PCO may mimic those of the primary cataract. Thus, PCO is often referred to as a “secondary cataract.” However, the visual impairment may be insignificant and thus require no intervention despite the clinical presence of PCO. A significant PCO requiring treatment may produce decreased visual acuity, glare, photophobia or impaired contrast, and color sensitivity. Patients generally notice a slow decline in visual quality after the initial visual improvement following cataract surgery.
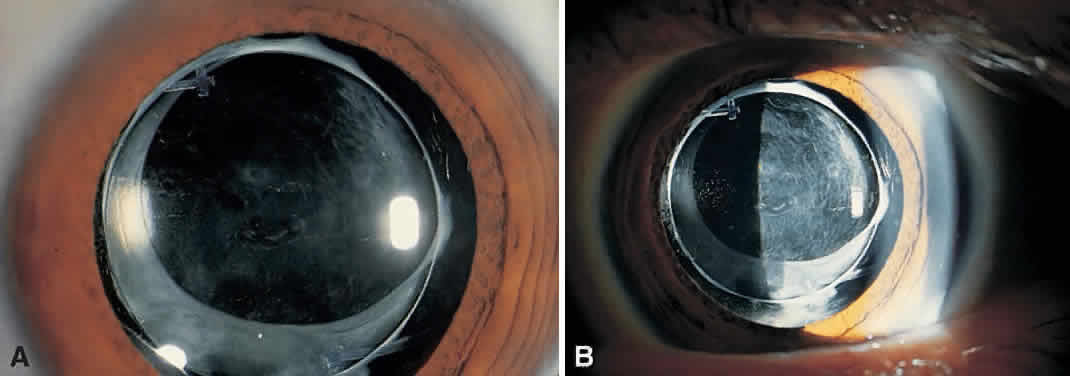
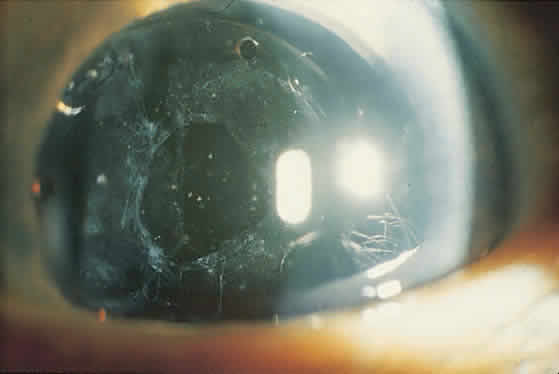
On clinical examination, retinoscopy identifies a red reflex that may be normal or slightly dull and irregular. Despite the ability to perform retinoscopy, the manifest refraction typically does not improve visual acuity. Slit lamp examination with side illumination demonstrates a thickened, irregular posterior capsule that may be slightly opacified or translucent (Fig. 1). Multiple vesicles in the capsule represent flattened Elschnig's pearls. Fluid in the vesicles is typically clear but may occasionally be turbid. Retroillumination may enhance the thickened, vesicular appearance of the posterior capsule. Examination of the retina is imperative to exclude other causes of decreased central vision that may occur after cataract surgery including cystoid macular edema, diabetic macular edema, or retinal detachment. Venous occlusive disease and age-related macular degeneration are additional causes of diminished visual acuity that commonly occur in this elderly population who undergo cataract surgery.
|
PROCEDURES
The Nd:YAG laser is a slit-lamp-mounted system with a double spot helium-neon aiming beam that merges to one beam with fine focusing. The eye is anesthetized topically and a contact lens maybe placed on the cornea if magnification of the posterior capsule is desired. The pupil may be pharmacologically dilated or remain undilated, depending on the preference of the treating ophthalmologist. Apraclonidine hydrochloride 1% is administered before laser treatment to prevent postoperative intraocular pressure (IOP) elevations. With the patient secured in the slit-lamp-mounted laser, the red helium-neon beam is focused on the posterior capsule so that energy is not transferred to the IOL or anterior hyaloid face. When properly focused, the two aiming beams merge into one. An initial laser power setting of 1.0 mJ or less is used to commence treatment. When Nd:YAG energy is successfully delivered to the posterior capsule, a break appears that enlarges along the lines of tension. Occasionally gas bubbles form on the capsule, which either clear spontaneously or can be dislodged by gently tapping on the contact lens. If the initial power setting fails to create an opening through the capsule, the energy level is gradually increased until laser disruption of the capsule occurs. Attempting to create a single opening in the capsule without increasing the energy level is ineffective and acts only to increase the total energy delivered to the eye. It is rarely necessary to increase the Nd:YAG laser power above 2.5 mJ.
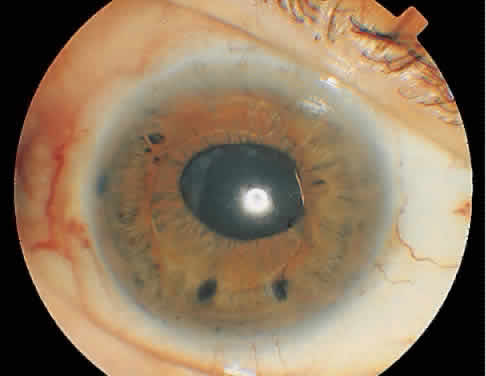
Multiple techniques exist to create an opening in the posterior capsule. These include cruciate, circular, or inverted U-shaped patterns. With the pupil in an undilated state, single shots are placed in the visual axis. Regardless of the technique, the goal is to create an opening in the posterior capsule that is slightly larger than the resting pupil (Fig. 2). Typically, this will be 3 to 4 mm and require 30 to 40 spots of laser to the posterior capsule. An effective way to minimize the number of laser applications is to treat the areas of capsule under tension. The openings created expand from the tensile forces of the opacified capsular bag. In a thickened opacified capsule, pieces of the capsule may be released into the anterior vitreous. These pieces inferiorly migrate out of the visual axis within 1 week of treatment without inducing any visual disturbances.
|
Most YAG capsulotomies are uncomplicated; an opening in the capsule is successfully created in 98% of cases.11 Visual acuity improves almost immediately following treatment. The eye may be treated before or after the procedure with apraclonidine 1% to blunt a potential rise in IOP, which is measured 20 to 60 minutes later. Persistent elevation of IOP occurs infrequently and is typically treated with an ocular hypotensive agent and close observation. Corticosteroid eye drops three or four times daily (e.g., fluorometholone acetate or prednisolone acetate 1%) for 4 to 7 days is a management option used by some ophthalmologists. Generally, patients experience only minimal symptoms, if any, after this procedure. Follow-up typically occurs in 1 week, unless the IOP is significantly elevated on post-YAG testing. Long-term follow-up is required in eyes with persistently elevated IOP.12
COMPLICATIONS
Although Nd:YAG laser posterior capsulotomy is a relatively straightforward procedure, complications may be encountered. The most common intraoperative complication reported in a cohort of 2110 patients followed by the Food and Drug Administration (FDA) was damage to the IOL.11 Accurate focusing of the helium-neon beam on the posterior capsule prevents the occurrence of this event. However, postoperative contraction of the capsular bag can move it into very close proximity to the posterior surface of the IOL, leading to this complication. Other factors that increase the likelihood of IOL damage include increased thickness of the PCO membrane and the total laser power used. Despite the occasional creation of laser-induced pits in the IOL, these typically do not cause visual disturbances. Rupture of the anterior hyaloid face was the second most frequently reported complication occurring in 19% of the patients observed during the FDA study. Less frequently encountered intraoperative complications were bleeding in 1.0%, corneal edema in 0.3%, and iris damage in 0.4% of cases.11
Elevation of IOP after Nd:YAG capsulotomy is the major postoperative complication encountered that requires treatment.11,13–15 The cause of this elevation is not certain but may be related to the release of lens epithelial cells into the anterior chamber. Variables such as the total energy delivered, number of laser pulses, size of the capsulot-omy, iris bleeding, and the amount of inflammation do not appear to affect IOP.14–16 However, some authors dispute this and assert that higher IOP after Nd:YAG capsulotomy is associated with larger capsulotomies and increased energy.12,13 In an FDA cohort of 213 patients following a protocol of measuring postprocedure IOP at selected intervals, 39% of patients had an increase of 5 mmHg or more in IOP 2 to 6 hours after laser capsulot-omy. About 28% experienced an increase in absolute IOP of more than 30 mm Hg. None of the eyes received prophylactic ocular hypotensive agents in the perioperative period.11 The maximum elevation of IOP occurred between 1.5 and 4 hours after laser treatment. Of these eyes, 60% returned to an IOP of less than 22 mmHg by 24 hours with 90% of eyes achieving normalization of IOP by 1 week.11 Results by Slomovic and Parrish15 mirrored the FDA's findings. Thus, 64% of eyes developed a maximum IOP rise by the second postoperative hour with 41% of eyes developing an IOP greater than 30 mm Hg. The results of the study of Slomovic and Parrish indicated that glaucomatous eyes, which were either medically or surgically controlled prior to capsulotomy, had a lower mean IOP rise after laser capsulotomy compared with eyes that had not been prophylactically treated.
With the discovery that pretreatment may blunt the postprocedure elevation in IOP, a prospective multicentered trial was initiated to evaluate the effect of apraclonidine applied in the perioperative period to treated eyes versus placebo. Apraclonidine 1% was administered 1 hour before and immediately after performing YAG laser capsulotomy.17 Placebo-treated eyes experienced a maximum IOP elevation of 4.4 mm Hg 3 hours after treatment. The IOP in apraclonidine-treated eyes decreased 2.8 mm Hg from baseline. As a result of the findings, eyes are now typically treated with apraclonidine or brimonidine when performing YAG laser capsulotomy.
Ge and colleagues12 evaluated long-term IOP fluctuations in eyes followed for a median of 1.5 years after YAG posterior capsulotomy. Eligible patients were pseudophakic bilaterally with one eye receiving laser capsulotomy and the other eye serving as a control. All eyes received apraclonidine before and after treatment. An elevated IOP mea-sured at 1 hour postprocedure was a significant risk factor for chronically increased IOP. This indicates that patients with elevated IOP after YAG laser capsulotomy despite pretreatment with ocular hypotensive agents should be monitored for persistent IOP elevation.
Other less common complications include retinal detachment and cystoid macular edema. In one recent evaluation of 196 eyes examined at 1 month after laser capsulotomy, only one eye sustained a new retinal break that was not demonstrated before treatment.18 Steinert and associates16 reviewed 879 Nd:YAG laser posterior capsulotomies over a 3-year period. Cystoid macular edema developed in 1.23% and retinal detachment occurred in 0.89%. Three of eight patients developed retinal detachment more than 1 year after laser capsulotomy. The number of laser pulses and amount of total energy delivered to the eye were not identified as risk factors. The FDA report of 2110 patients registered similar numbers.11 Cystoid macular edema occurred in 1.2% of the study eyes with retinal detachment in 0.5%. Less common complications included vitritis, iritis, and retinal hemorrhage in less than 0.6% of patients.11 Case reports of delayed posterior dislocation of silicone plate-haptic lenses have been described in the literature.19