| The first description of the astigmatic effect of nonpenetrating incisions
placed near the limbus dates back to 1898 and is credited to the Dutch
ophthalmologist L.J. Lans.30 As noted, LRIs have become the most popular technique employed today to
reduce pre-existing astigmatism at the time of implant surgery. Although
our preference is to use a temporal single-plane clear-corneal phaco
incision, one may utilize LRIs with any type of cataract incision
as long as the astigmatic effect is known and factored into the surgical
plan. LRIs offer several advantages over astigmatic incisions placed
within the cornea at smaller optical zones. These would include less
chance of causing a shift in the resultant cylinder axis. This presumably
is due to a diminished need for precise centration upon the steep
meridian. More importantly, there is less of a tendency to cause irregular
corneal flattening, and hence less chance of inducing irregular
astigmatism. Technically, LRIs are easier to perform and more forgiving
than shorter and more central corneal astigmatic incisions, and patients
generally report less discomfort. Another important advantage gained
by moving out to the limbus involves the “coupling ratio,” which
describes the amount of flattening that occurs in the incised
meridian relative to the amount of steepening that results 90 degrees
away. It has been our experience that paired LRIs (when kept at
or under 90 degrees of arc length) exhibit a very consistent 1:1 ratio, and
therefore elicit little change in spheroequivalence, obviating
the need to make any change in implant power. Admittedly, these more peripheral incisions are less powerful, but are
still capable of correcting up to 3 to 4 diopters of astigmatism in the
cataract-age population. One must keep in mind that the goal is to reduce
the patient's cylinder, without overcorrecting or shifting
the resultant axis. To achieve a given amount of correction, these peripheral
intralimbal incisions must be longer in total arc length than
more centrally-placed corneal astigmatic incisions; however, unlike longer
radial keratotomy incisions, they appear to be stable with regard
to refractive effect and show little sign of inducing problems such as
dry-eye syndrome or other pejorative effects from corneal denervation.22 Their stability may be due to the proximity of well-vascularized limbal
tissue. There are, of course, potential complications with any surgical
technique and these are addressed below. THE PLAN Perhaps the most challenging aspect of astigmatism surgery involves the
determination of the quantity and exact location of the cylinder that
is to be corrected, and thereby formulating a surgical plan. Unfortunately, preoperative
measurements—keratometry, refraction, and corneal
topography—do not always correlate. Lenticular astigmatism
may account for some of this disparity, particularly in cases where there
is a wide variance between refraction and corneal measurements; however, some
discrepancies are likely due to the inherent shortcomings
of traditional measurements of astigmatism. Standard keratometry, for
example, measures only two points in each meridian at a single optical
zone of approximately 3 mm. When confounding measurements do arise, one may compromise and average
the disparate readings; for example, if refraction shows 2 D of astigmatism
and keratometry reveals only 1 D, it would be reasonable to correct
for 1.5 D. Alternatively, if preoperative calculations vary widely, one
may defer placing the relaxing incisions until a stable refraction
postimplantation is obtained, and then correct any remaining astigmatism
as needed. LRIs may be safely performed in the office in an appropriate
treatment-room setting. Corneal topography can be very helpful
when refraction and keratometry do not agree, and it is increasingly
becoming the overall guiding measurement upon which the surgical plan
is based. Topography is also helpful in detecting subtle corneal pathology
such as keratoconus fruste, which would likely negate the use of
LRIs, or subtle irregular astigmatism such as that caused by epithelial
basement membrane dystrophy. NOMOGRAMS Once the amount of astigmatism to be corrected has been determined, a nomogram
must be consulted to determine the appropriate arc length of the
incisions. A number of popular nomograms are currently available.31 Our nomogram of choice originated from the work of Dr. Stephen Hollis
and incorporates concepts taught by Spencer Thornton, M.D., particularly
his age modifiers.24 As seen in the nomogram, a patient is considered to be “spherical” if
they have up to 0.75 D of with-the-rule or 0.50 D of against-the-rule
astigmatism, in which case a single plane temporal clear corneal
incision is used without additional wound manipulation (Table 1). If the patient has more than this amount of cylinder, one determines
whether it is WTR (45 to 135 degrees) or ATR (0 to 44 or 136 to 180 degrees) and then consults the appropriate section
of the nomogram. One aligns the patient's age with the amount
of preoperative cylinder to be corrected and finds the suggested arc
length that the incisions should subtend.
TABLE 1. Intralimbal Relaxing Incision Nomogram for Modern Phaco Surgery
| Surgery | Pre-op Cylinder | Paired Incisions in Degrees of Arc* | Incision design |
| 30 to 40 years old | 41 to 50 years old | 51 to 60 years old | 61 to 70 years old | 71 to 80 years old | 81 to 90 years old | 91+ years old |
| Sphericala | - | - | - | - | - | - | - | - | “Neutral” temporal clear corneal incision (i.e., ≤3.5 mm, single plane, just anterior to vascular arcade) |
| Against-the-ruleb | +0.75 to +1.25 | 55° | 50° | 45° | 40° | 35° | 35° | | The temporal incision, if greater than 40 degrees of arc, is made by first
creating a two-plane, grooved phaco incision (600 μm depth), which
is then extended to the appropriate arc length at the conclusion
of surgery. |
| +1.50 to +2.00 | 70° | 65° | 60° | 55° | 45° | 40° | 35° |
| +2.25 to +2.75 | 90° | 80° | 70° | 60° | 50° | 45° | 40° |
| +3.00 to +3.75 | 90° | 90° | 85° | 70° | 60° | 50° | 45° |
| With-the-rulec | +1.00 to +1.50 | 50° | 45° | 40° | 35° | 30° | | | “Neutral” temporal clear corneal along with the following peripheral
arcuate incisions |
| +1.75 to +2.25 | 60° | 55° | 50° | 45° | 40° | 35° | 30° |
| +2.50 to +3.00 | 70° | 65° | 60° | 55° | 50° | 45° | 40° |
| +3.25 to +3.75 | 80° | 75° | 70° | 65° | 60° | 55° | 45° |
Empiric blade-depth setting is 600 microns. When placing intralimbal relaxing
incisions following or concomitant with radial relaxing incisions, total
arc length is decreased by 50%.
*Nasal limbal arc
only.
aUp to +0.75 × 90 or +0.50 × 180.
bSteep Axis 0 to 44 degrees or 136 to 180 degrees.
cSteep Axis 45 to 135 degrees.
All incisions are paired, except in the case of very low ATR astigmatism
wherein a single 35-degree nasal LRI is placed opposite to the single-plane
temporal clear-corneal phaco incision. Paired incisions are preferred
to optimize symmetric corneal flattening and are expressed in
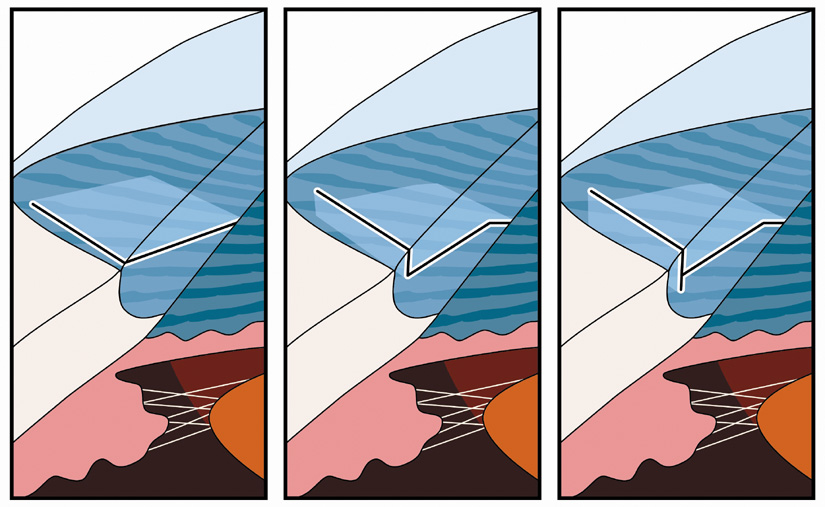
degrees of arc rather than chord length. This is done in order to diminish
over- and undercorrections for unusually small or large corneas, because
corneal diameter may significantly impact the relative length
of the arcuate incision and its resultant effect (Fig. 3). This nomogram, which has been designed specifically for the cataract
patient, is based upon the use of an empiric blade depth setting
of 600 microns. Individual surgeon technique and blade style may impact
results, and thereby require adjustment of the nomogram. A second, slightly
more aggressive nomogram is used with younger patients, particularly
in the setting of refractive lens exchange surgery or in conjunction
with LASIK for the correction of higher levels of astigmatism (Table 2). In this setting where optimal precision is mandated, pachymetry
is performed over the entire arc length of the intended incision site, and
a diamond blade with an adjustable micrometer is set to 90% of
the thinnest reading obtained. The NAPA nomogram, pachymetry, and
adjusted blade depth settings may certainly be used with the cataract
patient, but the small compromise that is made in using an empiric blade
depth setting is felt to be acceptable in this older patient population
in light of increased OR efficiency.
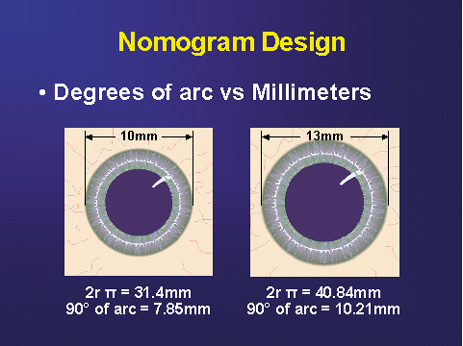 Fig. 3. Nomogram design. Note relative disparity in incision length between a large
and small corneal diameter if measured in millimeters. Degrees of
arc lend consistency irrespective of corneal size. (Reprinted from
Hardten DR, Lindstrom RL, Davis EA. Phakic Intraocular Lenses: Principles
and Practice. Thorofare, NJ: SLACK Incorporated, 2004, with permission.) Fig. 3. Nomogram design. Note relative disparity in incision length between a large
and small corneal diameter if measured in millimeters. Degrees of
arc lend consistency irrespective of corneal size. (Reprinted from
Hardten DR, Lindstrom RL, Davis EA. Phakic Intraocular Lenses: Principles
and Practice. Thorofare, NJ: SLACK Incorporated, 2004, with permission.)
|
TABLE 2. Nichamin Age & Pachymetry-Adjusted (NAPA) Intralimbal
Arcuate Astigmatic Nomogram
| Surgery | Pre-op cylinder (diopters) | Paired Incisions in Degrees of Arc |
| 20 to 30 years old | 31 to 40 years old | 41 to 50 years old | 51 to 60 years old |
| With-the-rulea | 0.75 | 40° | 35° | 35° | 30° |
| 1.00 | 45° | 40° | 40° | 35° |
| 1.25 | 55° | 50° | 45° | 40° |
| 1.50 | 60° | 55° | 50° | 45° |
| 1.75 | 65° | 60° | 55° | 50° |
| 2.00 | 70° | 65° | 60° | 55° |
| 2.25 | 75° | 70° | 65° | 60° |
| 2.50 | 80° | 75° | 70° | 65° |
| 2.75 | 85° | 80° | 75° | 70° |
| 3.00 | 90° | 90° | 85° | 80° |
| Against-the-ruleb | 0.75 | 45° | 40° | 40° | 35° |
| 1.00 | 50° | 45° | 45° | 40° |
| 1.25 | 55° | 55° | 50° | 45° |
| 1.50 | 60° | 60° | 55° | 50° |
| 1.75 | 65° | 65° | 60° | 55° |
| 2.00 | 70° | 70° | 65° | 60° |
| 2.25 | 75° | 75° | 70° | 65° |
| 2.50 | 80° | 80° | 75° | 70° |
| 2.75 | 85° | 85° | 80° | 75° |
| 3.00 | 90° | 90° | 85° | 80° |
When placing intralimbal relaxing incisions following or concomitant with
radial relaxing incisions, total arc length is decreased by 50%.
aSteep axis 45 to 135 degrees.
bSteep axis 0 to 44 degrees or 136 to 180 degrees.
SURGICAL TECHNIQUE Some surgeons prefer to perform LRIs at the conclusion of surgery in the
event that a complication occurs necessitating a modification to the
phaco incision. For routine cases, however, our preference is to place
these relaxing incisions at the outset of surgery in order to minimize
epithelial disruption. The one exception to this rule occurs in the
case of high ATR astigmatism wherein the nomogram calls for a temporal
arcuate incision greater than 40 degrees of arc. Because the temporal
arc will be superimposed upon the phaco incision, if it is extended
to its full arc length at the start of surgery, significant gaping and
edema may result secondary to intraoperative wound manipulation. In this
setting, the temporal incision is first made by creating a two-plane
grooved phaco incision (at 600 micron depth). Following IOL
implantation and prior to viscoelastic removal, while the globe is
still firm, the relaxing incision is extended to its full arc length as
dictated by the nomogram. When an LRI is superimposed upon the phaco
tunnel, the keratome entry is first accomplished by pressing the bottom
surface of the keratome blade downward upon the outer or posterior
edge of the LRI. The keratome is then advanced into the LRI at an iris-parallel
plane. This angulation will promote a dissection that takes
place at midstromal depth, which will help assure adequate tunnel length
and a self-sealing closure. Proper centration of the incisions over the steep corneal meridian is of
utmost importance. Increasing evidence supports the notion that significant
cyclotorsion may occur when assuming a supine position.32 As previously noted, an axis deviation of only 15 degrees may result in
a 50% reduction of surgical effect.5 For this reason, most surgeons advocate placing an orientation mark at
the 12:00 or 6:00 limbus while the patient is in an upright position. This
is particularly important when employing injection anesthesia wherein
unpredictable ocular rotation may occur. An additional measure that
may be employed to help center the relaxing incisions is to identify
the steep meridian (plus cylinder axis) intraoperatively
using some form of keratoscopy. The steep meridian over which the incisions
are to be placed corresponds to the shorter axis of the reflected
corneal mire. A simple handheld device such as the Maloney (Storz, Katena) or
Nichamin (Mastel Precision) keratoscope
works well, or a more robust and well-defined mire may be obtained through
an elaborate microscope-mounted instrument such as the Mastel Ring
of Light (Mastel Precision). Another common way in which the
steep meridian is marked utilizes a Mendez Ring or similar degree gauge
that is aligned with the previously placed limbal orientation mark, and
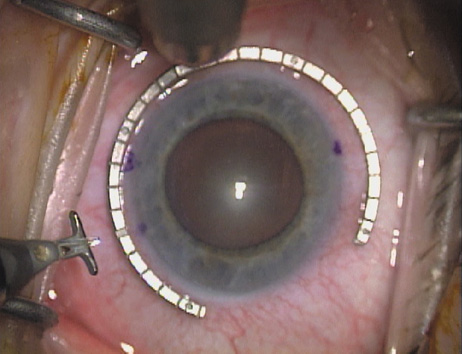
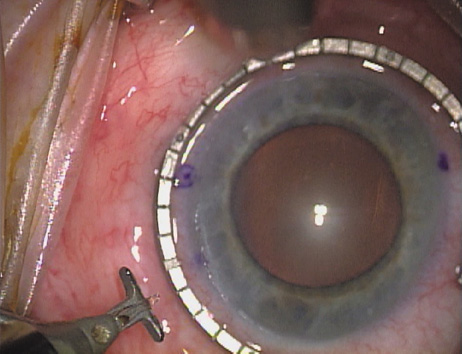
the cylinder axis is then located on the 360-degree gauge. The LRI should be placed at the most peripheral extent of clear corneal
tissue, just inside of the true surgical limbus. This holds true irrespective
of the presence of pannus or blood vessels. If bleeding occurs, it
may be ignored and will cease spontaneously. One must avoid placing
the incisions further out at the true surgical limbus in that a significant
reduction of effect will likely occur due to both increased
tissue thickness and a variation in tissue composition; these incisions
are, therefore, really intralimbal in nature. In creating the incision, it is important to hold the
knife perpendicular to the corneal surface in order to achieve consistent
depth and effect. Good hand and wrist support is important, and the
blade ought to be held as if one were throwing a dart such that the
instrument may be rotated between thumb and index finger as it is being
advanced, thus leading to smooth arcuate incisions. Typically, the
right hand is used to create incisions on the right side of the globe, and
the left hand for incisions on the left side. In most cases, it is
more efficient to pull the blade toward oneself, as opposed to pushing
it away. A lightly moistened corneal surface will help to prevent epithelial
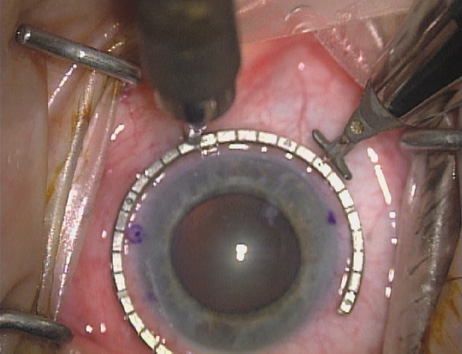
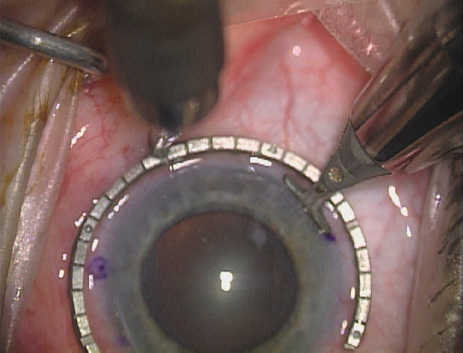
disruption, but may mask an unintentional perforation. The extent of arc to be incised may be demarcated in several different
ways. Our preferred method makes use of a modified Fine-Thornton fixation
ring (Nichamin Fixation Ring and Gauge; Mastel Precision, Storz, Rhein
Medical). This instrument serves to fixate and position
the globe in order to optimize incision placement, as well as to delineate
the extent of arc to be incised. One visually extrapolates from
the limbus to marks on the surface of the ring. Each incremental mark
is 10 degrees apart, and bold hash marks (180 degrees) opposite
to each other serve to align and center the incision over the steep
meridian. This approach obviates the need to ink and physically mark
the cornea. If one desires, particularly when first gaining experience
with LRIs, a two-cut RK marker may be used to place ink marks upon the
cornea to show the exact extent of arc that is to be incised, in conjunction
with the fixation ring/gauge (Fig. 4). Alternatively, various press-on markers are available, such as
those made by Rhein Medical (Dell-Nichamin Marker, Nichamin-Kershner
Marker, or the Ruminson Marker) (Fig. 5). ASICO and other instrument companies offer a full line of dedicated
markers, rings, and blades for performing LRIs. 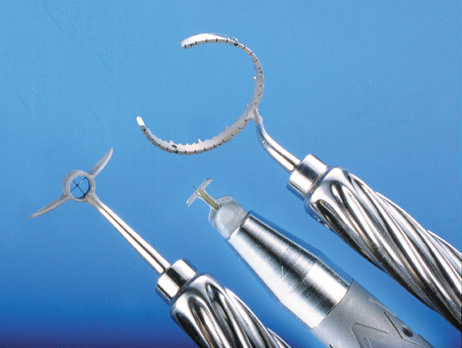 Fig. 4. The Nichamin Fixation Ring and Gauge serves to both fixate the globe and
delineate the extent of arc to be incised; a two-cut radial marker may
be used to mark the extent of arc to be incised, and the Mastel Nichamin
Force AK Diamond Blade with preset depth of 600 microns. Fig. 4. The Nichamin Fixation Ring and Gauge serves to both fixate the globe and
delineate the extent of arc to be incised; a two-cut radial marker may
be used to mark the extent of arc to be incised, and the Mastel Nichamin
Force AK Diamond Blade with preset depth of 600 microns.
|
As noted, in the setting of concomitant cataract surgery, an empiric blade
depth setting of 600 microns is commonly employed. Various knives
have been designed specifically for this application, ranging from disposable
steel blades to exquisite gemstone diamond knives. Synthetic (and
less expensive) diamond materials are also available and
are intended for limited reuse. Our preference is for diamond blade technology
that incorporates a single small and arced footplate for enhanced
visualization at the limbus (Mastel Precision). Two models
are available, one with a preset depth of 600 microns and the other
with an adjustable micrometer handle (Fig. 6). Similar designs are available from Rhein Medical, Storz, ASICO, and
other manufacturers.   Fig. 6. (A)A diamond blade with a preset depth of 600 microns is used
to perform LRIs for routine cataract surgery. (B) An adjustable
depth micrometer blade is used in conjunction with the NAPA nomogram
when treating younger patients. Fig. 6. (A)A diamond blade with a preset depth of 600 microns is used
to perform LRIs for routine cataract surgery. (B) An adjustable
depth micrometer blade is used in conjunction with the NAPA nomogram
when treating younger patients.
|
Another less common method of creating peripheral relaxing incisions is
to use a device such as the Terry/Schanzlin Astigmatome (Oasis
Medical), which circumvents the need to make a “free-hand” incision. This
trephine-like device has been designed to create
consistent and symmetric peripheral arcuate corneal relaxing incisions. It
uses a vacuum speculum that mates with various reusable templates
that are selected based upon the amount of astigmatic correction that
is desired. The incision is created by simply rotating a disposable
steel blade unit that fits inside of the template (Fig. 7). 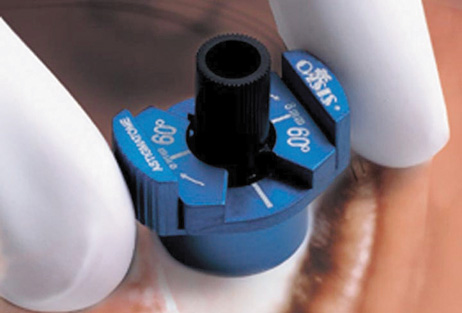 Fig. 7. The Terry/Schanzlin Astigmatome. Fig. 7. The Terry/Schanzlin Astigmatome.
|
COMPLICATIONS As discussed, LRIs are proving to be a safer and more forgiving approach
to managing astigmatism as compared to more central corneal incisions. Nonetheless, as
with any surgical technique, potential complications
exist, and several are listed in Table 3. Of these, the most likely to be encountered is the placement of incisions
upon the wrong axis. When this occurs, it typically takes the form
of a 90-degree error with positioning upon the opposite, flat meridian. This, of
course, results in an increase and likely doubling of the
patient's preexisting cylinder. Compulsive attention is required
in this regard. The surgeon ought to consider employing safety checks
to prevent this frustrating complication from occurring, such as having
a written plan that is brought into the operating room, kept visible
and properly oriented. Incisions are always placed upon the plus (+) cylinder
axis and opposite to the minus (−) cylinder
axis.
TABLE 3. Potential Problems
| Infection |
| Weakening of the globe |
| Perforation |
| Decreased corneal sensation |
| Induced irregular astigmatism |
| Misalignment/axis shift |
| Wound gape and discomfort |
| Operating upon the wrong (opposite) axis! | Although very rare when utilizing a blade depth setting of 600 microns, corneal
perforation is possible. This may be due to improper setting
of the blade depth or as a result of a defect in the micrometer mechanism. This
latter problem may arise after repeated autoclaving and many
sterilization runs. Periodic inspection and calibration is therefore
warranted, even with preset single depth knives. When encountered, unlike
radial microperforations, these circumferential perforations will
rarely self-seal and will likely require placement of temporary sutures. ENHANCEMENT TECHNIQUES As mentioned, LRIs lend themselves well to in-office “touch-ups.” Although
some surgeons will place or extend incisions at the slit-lamp, it
is our preference to use a small operating microscope and
to perform the procedure within a dedicated treatment room. It has been
our experience that this provides far better surgical control as well
as patient comfort. In the case of residual astigmatism without prior
incisional correction, one uses the same technique and nomogram as described
above. In the case of an undercorrection following previous LRIs, one should inspect
the length and positioning of the incisions. As indicated, placement
of the incisions too far out into the true surgical limbus and beyond
clear cornea will often lead to undercorrection. If the arc length
and location appear to be adequate, one ought to suspect that the patient
has an unusually thick cornea. This occurs most frequently in hyperopic
eyes. In this situation, pachymetry should be performed and the
incisions may be redeepened or extended. When faced with an overcorrection, one
should resist the temptation to place additional incisions
in the opposite meridian. This can lead to an unstable cornea with unpredictable
refractive results, or worse, induce irregular astigmatism. Rather, one
should consider nonincisional modalities such as PRK or
LASIK. We also have had good results in this setting using conductive
keratoplasty off-label, particularly if the overcorrection involves hyperopic
astigmatism.29 To correct unusually high levels of astigmatism, LRIs may be used in conjunction
with a toric IOL or excimer laser surgery (bioptics). In
several rare cases, we have combined all three modalities and safely
corrected up to 9 D of preexisting astigmatism! |