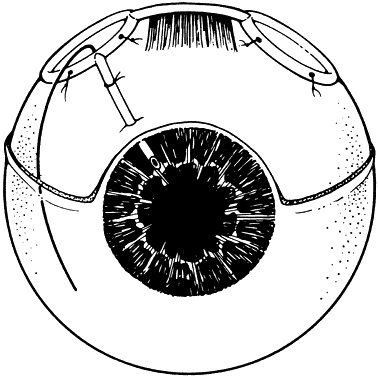
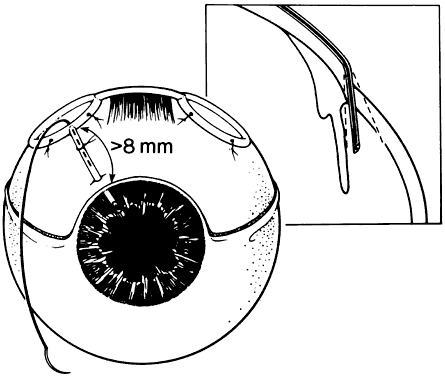
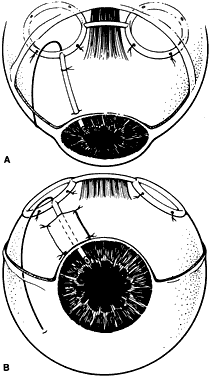
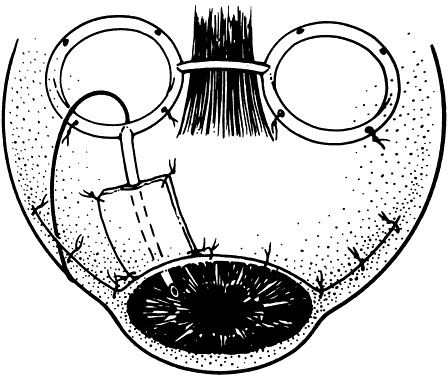
1. Katz LJ, Spaeth GL: Surgical management of the secondary glaucomas. Part 1. Ophthalmol Surg 18:826, 1987 2. Eid TE, Katz LJ, Spaeth GL et: Tube-shunt surgery versus neodymium:YAG
cyclophotocoagulation in the management of neovascular glaucoma. Ophthalmology 104:1692, 1997 3. Rollet M: Le drainage au irin de la chambre anterieure contre l'hypertonie et al
douleur. Rev Gen Ophthalmol 25:481, 1906 4. Zorab A: The reduction of tension in chronic glaucoma. Ophthalmolscope 10:258, 1912 5. Stefansson J: An operation for glaucoma. Am J Ophthalmol 8:681, 1925 6. Muldoon WE, Ripple PH, Wilder HC: Platinum implant in glaucoma surgery. Arch Ophthalmol 45:666, 1951 7. Troncoso MU: Use of tantalum implants for inducing a permanent hypotony in rabbit eyes. Am J Ophthalmol 32: 499, 1949 8. Bock RH: Subconjunctival draining of the anterior chamber by a glass seton. Am J Ophthalmol 33:929, 1950 9. Stewart RH, Kimbrough RL, Okercke PC: Trabeculectomy with implantation of the Mendez glaucoma section: Early
results. Ophthalmol Surg 17:221, 1986 10. LaRocca V: Gonioplasty in glaucoma. Br J Ophthalmol 46: 404, 1962 11. Honrubia FM, Gomez ML, Hernandez A et al: Long term results of silicone tube in filtering surgery for eyes with neovascular
glaucoma. Am J Ophthalmol 97:501, 1984 12. Igerer I: Silicone catheters used as setons in glaucoma surgery. Glaucoma 5:32, 1983 13. Kalijaca Z, Ljubojevic V, Manirov D: Draining implant for neovascular glaucoma. Am J Ophthalmol 96:372, 1983 14. Refojo MF: Current status of biomaterials in ophthalmology. Surv Ophthalmol 26:257, 1982 15. Epstein E: Fibrosis response in aqueous. Br J Ophthalmol 43:641, 1959 16. Richards RD, Van Bijsterveld OP: Artificial draining tube for glaucoma. Am J Ophthalmol 60:405, 1965 17. Krupin T, Kaufman P, Mandell A et al: Filtering valve implant surgery for eyes with neovascular glaucoma. Am J Ophthalmol 89:338, 1980 18. Sutton GE, Popp JC, Records RF: Krupin-Denver valve and neovascular glaucoma. Trans Ophthalmol Soc UK 102: 119, 1982 19. Folberg R, Hargett NA, Weaver JE et al: Filtering valve implant for neovascular glaucoma in proliferative diabetic
retinopathy. Ophthalmology 89:286, 1982 20. Molteno ACB: New implant for draining in glaucoma. Br J Ophthalmol 53:609, 1969 21. Molteno ACB, Van Roogen MMB, Bartholomew RS: Implants for draining neovascular glaucoma. Br J Ophthalmol 61:120, 1977 22. Schocket SS, Nirankari VS, Lakhampal V et al: Anterior chamber tube shunt to an encircling band in the treatment of neovascular
glaucoma and other refractory glaucomas. Ophthalmology 92:553, 1985 23. Krupin T, Ruderman JR, Rosenberg LE et al: Glaucoma valve to an external disc implant for filtration surgery. Invest Ophthalmol Vis Sci 33:948, 1992 23a. Coleman AL, Hill R, Wilson MR et al: Initial clinical experience with the Ahmed glaucoma valve implant. Am J Ophthalmol 120:23, 1995 24. Carmeron JD, White TC: Clinico-histopathologic correlation of a successful glaucoma pump shunt
implant. Ophthalmology 95:1189, 1988 25. Minckler DS, Shammas A, Wilcox, M et al: Experimental studies of aqueous using the Molteno seton. Trans Am Ophthalmol Soc 85:385, 1987 26. Rubin B, Chan CC, Burnier M et al: Histopathologic study of the Molteno glaucoma implant in three patients. Am J Ophthalmol 110:371, 1990 27. Loeffler KU, Jay JL: Tissue response to aqueous drainage in a functioning Molteno implant. Br J Ophthalmol 72:29, 1988 28. Schocket SS: Investigations of the reasons for success and failure in the anterior shunt
to the encircling band procedure in the treatment of refractory glaucoma. Trans Am Ophthalmol Soc 84:743, 1986 29. Lloyd MA, Baerveldt G, Quang HN et al: Long-Term histologic studies of the Baerveldt implant in a rabbit model. J Glaucoma 5:334, 1996 30. Folberg R, Hargett NA, Weaver JE et al: Filtering valve implants for neovascular glaucoma and proliferative diabetic
retinopathy. Ophthalmology 89:286, 1982 31. Anker E, Molteno ACB: Molteno drainage implant for neovascular glaucoma. Trans Opthalmol Soc UK 102: 122, 1982 32. Brown RD, Cairns JE: Experience with the Molteno long tube implant. Trans Ophthalmol Soc UK 103:297, 1983 33. Freedman J: The use of single stage Molteno long tube seton in treating resistant cases
of glaucoma. Ophthalmolic Surg 16:480, 1985 34. Hoare Nairne JEA, Sherwood D, Jacob JSH, Rich WJCC: Single stage insertion of the Molteno tube for glaucoma and methods to
reduce postoperative hypotony. Br J Ophthalmol 72:846, 1988 35. Latina MA: Single stage Molteno implant with combination internal occlusion and external
ligature. Ophthalmol Surgery 12(6):444, 1990 36. Minckler DS, Baerveldt G, Heuer DK: Clinical experience with the Molteno implant in complicated glaucoma cases. Ophthalmology 95:1181, 1988 37. Molteno ACB: The use of drainage implants in resistant cases of glaucoma. Late results
of 110 operations. Trans Ophthalmol Soc NZ 32:101, 1983 38. Hill RA, Heuer DK, Baerveldt G et al: Molteno implantation for glaucoma in young patients. Ophthalmology 98: 1042, 1991 39. Fish L, Heuer DK, Baerveldt G: Molteno implantation for secondary glaucomas associated with advanced epithelial
ingrowth. Ophthalmology 97:557, 1990 40. Freedman J, Rubin B: Molteno implants as a treatment for refractory glaucoma in black patients. Arch Ophthalmol 109:1417, 1991 41. Traverso CE, Tomey KF, Al-Kaff A: The long-tube single plate Molteno implant for the treatment recalcitrant
glaucoma. Int Ophthalmol 13(1–2):159, 1989 42. Wilson RP, Cantor L, Katz, LJ: Aqueous shunts. Ophthalmology 99:672, 1992 43. Smith MF, Sherwood MB, McGorrary MB: Comparison of the double plate Molteno drainage implant with the Schocket
procedure. Arch Ophthalmol 40:1246, 1992 44. Camras CB: Discussion of paper by Wilson RP et al: Aqueous shunts: Molteno
vs. Schocket. Ophthalmology 99:676, 1992 45. Heuer DK, Lloyd MA, Abrams DA et al: Preliminary report of a randomized
clinical trial of single plate versus double plate Molteno implantation
for glaucoma in aphakia and pseudophakia. In Kriegelstein GK (ed): Glaucoma
Update IV, pp 224–249. Berlin, Springer-Verlag, 1991 46. Wilson RP: The Schocket shunt. Ophthalmology 1:225, 1988 47. Omi CA, DeAlmeida GV, Cohen R et al: Modified Schocket implant for refractory glaucoma. Ophthalmology 98:211, 1991 48. Krupin T, Ritch R, Camras CB et al: A long Krupin-Denver valve implant attached to a 180° scleral explant
for glaucoma surgery. Ophthalmology 95:1174, 1988 49. Saravitz EM, Toris CB, Camras CB et al: Opening/closing pressures and flow rates of Krupin-Denver valves in air
and/or water. Invest Ophthalmol Vis Sci 33:948, 1992 50. Hitchings RA, Joseph NH, Sherwood MD et al: Use of one-piece valved tube and variable surface area explant for glaucoma
drainage surgery. Ophthalmology 94:1079, 1987 51. Hitchings RA, Lavin MJ, Calthrope M: Glaucoma drainage tubes. Their role in glaucoma management. Int Ophthalmol 13:151, 1987 52. Lavin MJ, Franks WA, Wormaid RPL, Hitchings RA: Clinical risk factors for failure in glaucoma tube surgery. Arch Ophthalmol 110:480, 1992 53. Baerveldt G, Heuer DK, Martone JF et al: Clinical experience with the Baerveldt
implant in complicated glaucomas. Personal communication, 1992 54. Smith SL, Starita RJ, Fellman RL et al: Extraocular muscle imbalance after anterior chamber tube shunt to the Baerveldt 350 mm2 Implant. Invest Ophthalmol Vis Sci 33: 949, 1992 55. Ohdara E, Kubota H, Takanashi T et al: Outcome of White pump shunt surgery for neovascular glaucoma in Asians. Ophthalmol Surg 23:666, 1992 56. Davidovski F, Stewart RH, Kimbrough RL: Long-term results with the White glaucoma pump shunt. Ophthalmol Surg 21:288, 1990 57. Ayyala RS, Zurakowski D, Smith JA et al: A clinical study of the Ahmed glaucoma valve implant in advanced glaucoma. Ophthalmology 105:1968, 1998 58. Prata JA Jr, Mermoud A, LaBree L et al: In vitro and in vivo flow characteristics of glaucoma drainage implants. Ophthalmology 102:894, 1995 59. Porter JM, Krawezyk CH, Carey RF: In vitro flow testing of glaucoma drainage devices. Ophthalmology 104:1701, 1997 60. Francis BA, Cortes A, Chen J et al: Characteristics of glaucoma drainage implants during dynamic and steady-state
flow conditions. Ophthalmology 105:1708, 1998 61. Prata JA Jr, Minckler DS, Mermoud A et al: Effects of Intraoperative mitomycin-C on the function of Baerveldt glaucoma
drainage implants in rabbits. J Glaucoma 5:29, 1996 62. Lee D, Shin DH, Birt CM et al: The effect of adjuctive mitomycin-C in Molteno implant surgery. Ophthalmology 104:2126, 1997 63. Lloyd MA, Heuer DK, Baerveldt G et al: Combined Molteno implantation and pars plana vitrectomy for neovascular
glaucomas. Ophthalmology 98:1401, 1991 64. Gandham SB, Costa VP, Katz LJ et al: Aqueous tube-shunt implantation and pars plana vitrectomy in eyes with
refractory glaucoma. Am J Ophthalmol 116:189, 1993 65. Fish LA, Heuer DK, Baerveldt G et al: Molteno implantation for secondary glaucomas associated with advanced epithelial
ingrowth. Ophthalmology 97:557, 1990 66. Costa VP, Katz LJ, Cohen EJ, Raber IM: Glaucoma associated with epithelial downgrowth controlled with Molteno
tube shunts. Ophthalmol Surg 23:797, 1992 67. McDonnell PJ, Rubin JB, Schanzlin DJ: Molteno implant for control of glaucoma in eyes after penetrating keratoplasty. Ophthalmology 93:364, 1988 68. Beebe W, Starita R, Fellman R et al: The use of Molteno implant and anterior chamber tube shunt to encircling
band for the treatment of glaucoma in keratoplasty patients. Ophthalmology 97:1414, 1990 69. Billson F, Thomas R, Aylward W: The use of two stage Molteno implants in developmental glaucoma. J Pediatr Ophthalmol Strabismus 26:3, 1989 70. Munoz M, Tomey K, Traverso C et al: Clinical experience with the Molteno implant in advanced infantile glaucoma. J Pediatr Ophthalmol Strabismus 28:68, 1991 71. Hill RA, Heuer OK, Baerveldt G et al: Molteno implantation for glaucoma in young patients. Ophthalmology 98: 1042, 1991 72. Eid TE, Katz LJ, Spaeth GL et al: Long-term effects of tube shunt procedures on refractory childhood glaucoma. Ophthalmology 104:1011, 1997 73. Fellenbaum PS, Sidoti PA, Heuer DK et al: Experience with the Baerveldt implant in young patients with complicated
glaucomas. J Glaucoma 4:91, 1995 74. Weiss HS: Postoperative manipulation of the Krupin valve. Ophthalmic Surg Lasers 27:151, 1996 75. Burchfield JC, Kass MA, Wax MB: Primary valve malfunction of the Krupin eye valve with disk. J Glaucoma 6: 152, 1997 76. Feldman RM, El-Harazi SM, Villanueva G: Valve membrane adhesion as a cause of Ahmed glaucoma valve failure. J Glaucoma 6:10, 1997 77. Azuara-Blanco A, Katz LJ, Gandham SB et al: Pars plana tube insertion of aqueous shunt with vitrectomy in malignant
glaucoma. Arch Ophthalmol 116:808, 1998 78. Canning CR, Lavin M, McCartney AC: Delayed suprachoroidal hemorrhage after glaucoma operations. Eye 3:327, 1989 79. Franks WA, Hitchings RA: Injection of perfluoropropane gas to prevent hypotony in eyes undergoing
tube implant surgery. Ophthalmology 97:899, 1990 80. Kooher JS: Repair of Mottino implant during surgery. Am J Ophthalmol 117:673, 1994 81. Molteno ACB, Van Bijon G, Anker E: Two stage insertion of glaucoma drainage implants. Trans Ophthalmol Soc NZ 31:17, 1979 82. Molteno ACB, Polkinghorne PJ, Bowbyes JA: the Vieryl tie technique for inserting a draining implant in the treatment
of secondary glaucoma. Aust NZ J Ophthalmol 14: 343, 1986 83. El Sayyad F, El-Maghraby A, Hetal M et al: The use of releasable sutures in Molteno glaucoma implant procedures to
reduce postoperative hypotony. Ophthalmol Surg 22: 82, 1991 84. Price FW Jr, Whitsen WE: Polypropylene ligatures as a means of controlling intraocular pressure
with Molteno implants. Ophthalmol Surg 20:781, 1989 85. Liebmann J, Ritch R: Intraocular suture ligature to reduce hypotony following Molteno seton
implantation. Ophthalmol Surg 23:51, 1992 86. Traverso CF, Tomey KF, Al-Katita A: The long tube single plate Molteno implant for the treatment of recalcitrant
glaucoma. Int Ophthalmol 13:159, 1989 87. Latina M: Single stage Molteno implant with combination internal occlusion and external
ligature. Ophthalmol Surg 21:444, 1990 88. Kooner KS, Goode SM: Removable ligature during Molteno implant procedure. Am J Ophthalmol 114:102, 1992 89. Susanna R Jr: Modifications of the Molteno implant and implant procedure. Ophthalmol Surg 22:611, 1991 90. Egbert PR, Lieberman MP: Internal suture occlusion of the Molteno glaucoma implant for the prevention
of postoperative hypotony. Ophthalmol Surg 20:53, 1989 91. Molteno ACB: The dual chamber implant: its use in neovascular glaucoma. Aust NZ J Ophthalmol 18:131, 1990 92. Freedman J: Clinical experience with the Molteno dual-chamber single-plate implant. Ophthalmol Surg 23:238, 1992 93. Chen PP, Palmberg PF: Needling revision of glaucoma drainage device filtering blebs. Ophthalmology 104:1004, 1997 94. Ayyala RS, Harman LE, Michelini-Norris B et al: Comparison of different biomaterials for glaucoma drainage devices. Arch Ophthalmol 118:233, 1999 95. Valimaki J, Tuulonen A, Airaksinen PJ: Capsule excision after failed Molteno surgery. Ophthalmic Surg Lasers 28: 382, 1997 96. Mills RP, Reynolds A, Emond M et al: Long-term survival of Molteno glaucoma drainage devices. Ophthalmology 103:229, 1996 97. Fiore PM, Melamed S: Use of neodymium:YAG laser to open an occluded Molteno tube. Ophthalmol Surg 20: 373, 1989 98. Singh K, Eid TE, Katz LJ et al: Evaluation of Nd:YAG laser membranectomy in blocked tubes after glaucoma
tube-shunt surgery. Am J Ophthalmol 124:781, 1997 99. Zalloum JN, Ahuja RM, Shin D et al: Assessment of corneal decompensation in eyes having undergone Molteno shunt
procedures compared to eyes having undergone trabeculectomy. CLAO J 25:57, 1999 100. Billson F, Thomas R, Grigg J: Resiting Molteno tubes. Ophthalmic Surg Lasers 27:801, 1996 101. Allinson RW: Paralimbal compression suture for Molteno implants. Ophthalmol Surg 22:750, 1991 102. Rapuano CJ, Schmidt CM, Cohen EJ et al: Results of alloplastic tube shunt procedures before, during, or after penetrating
keratoplasty. Cornea 14:26, 1995 102a. Christmann LM, Wilson ME: Motility disturbances after Molteno implants. J Pediatr Ophthalmol Strabismus 29: 44, 1992 103. Ball S, Ellis GS, Herrington RG et al: Brown's superior oblique tendon syndrome after Baerveldt glaucoma
implant. Arch Ophthalmol 110:1368, 1992 104. Ball SF, Lotfield K, Scharfenberg J: Molteno rip-cord suture hypopyon. Ophthalmol Surg 21:407, 1990 105. Perkins T: Endophthalmitis after placement of a Molteno implant. Ophthalmol Surg 21:733, 1990 106. Krebs DB, Liebmann JM, Ritch R et al: Late infectious endophthalmitis from exposed glaucoma setons. Arch Ophthalmol 110:174, 1992 107. Melamed S, Cahane M, Gutman I et al: Postoperative complications after Molteno implant surgery. Am J Ophthalmol 111:319, 1991 108. Lotufo DG: Postoperative complications and visual loss following Molteno implantation. Ophthalmol Surg 22: 650, 1991 109. Kramer T, Brown R, Lynch M et al: Molteno implants and operating microscope-induced retinal phototoxicity. Arch Ophthalmol 109:379, 1991 110. Huna R, Melamed S, Hirsh A et al: Retinal detachment adherent to posterior chamber IOL after Molteno implant
surgery. Ophthalmol Surg 21:854, 1990 |