INTRAOPERATIVE AND POSTOPERATIVE SUPRACHOROIDAL HEMORRHAGE
Suprachoroidal hemorrhage is a serious complication that can be seen during or after any intraocular surgery. If it occurs intraoperatively and cannot be controlled (i.e., expulsive hemorrhage), it can lead to loss of vision. The incidence of this complication in the general population after cataract extraction is approximately 0.2%.1–3 The incidence of suprachoroidal hemorrhage in glaucoma patients undergoing various types of intraocular surgery has been reported to be 0.73%.4–6 Ocular risk factors for suprachoroidal hemorrhage include glaucoma, aphakia, pseudophakia, previous vitrectomy, vitrectomy at the time of glaucoma surgery, myopia, and postoperative hypotony. Systemic risk factors are arteriosclerosis, high blood pressure, tachycardia, and bleeding disorders. The source of the hemorrhage usually is one of the posterior ciliary arteries, particularly the point of entrance of the short posterior ciliary vessels into the suprachoroidal space. There seems to be a vascular necrosis and subsequent rupture of the vascular wall.7
Intraoperative suprachoroidal hemorrhage can be associated with sudden collapse of the anterior chamber. The patient may complain of sudden pain breaking through the local anesthesia. If the process is gradual, a dark mass can be observed through the pupil to evolve slowly, but if the process is abrupt the hemorrhage is more expulsive.
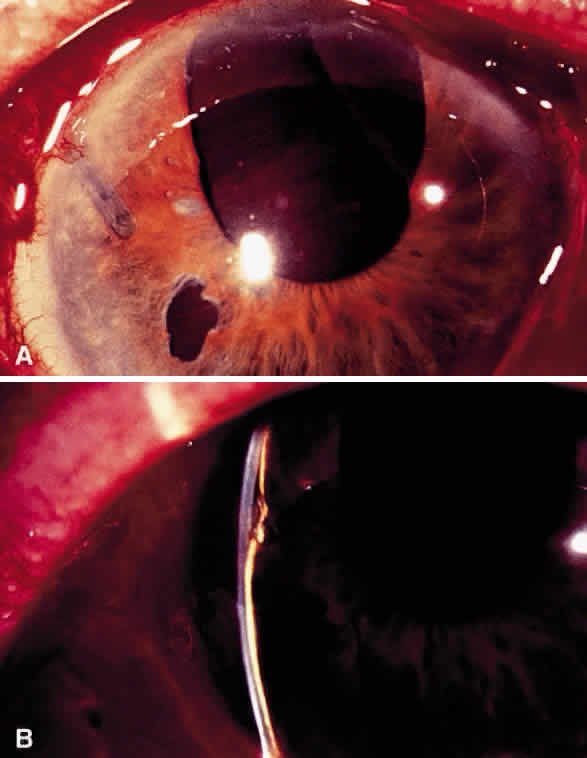
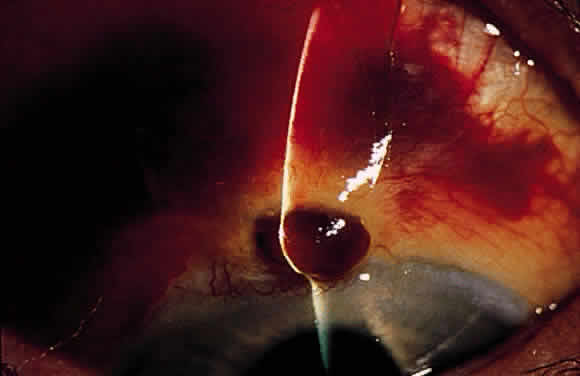
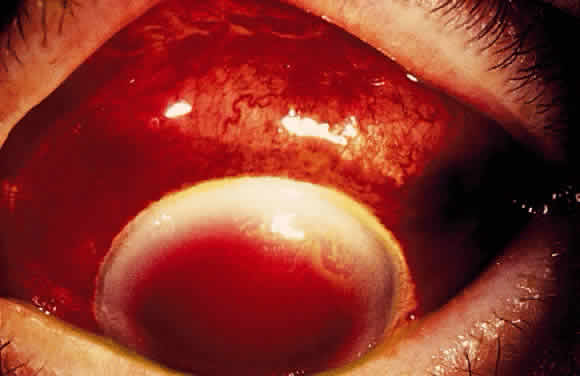
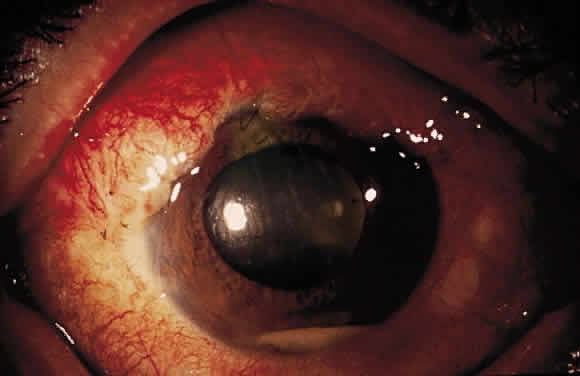
Postoperative suprachoroidal hemorrhage usually occurs within the first week after glaucoma surgery and usually is associated with postoperative hypotony4–6 (Fig. 1). The development of a suprachoroidal hemorrhage typically is acute and associated with the sudden onset of severe pain. Examination of the anterior segment frequently reveals a shallow anterior chamber and a normal or high intraocular pressure (IOP). On fundus examination, a detached and dark choroid is noted. The choroidal elevations have a dark, reddish brown color. Some patients present with bleeding into the vitreous cavity and, uncommonly, retinal detachment. Ultrasonography can be used to diagnose suprachoroidal hemorrhage when fundus examination is not possible.
Intraoperatively, once a suprachoroidal hemorrhage has been identified, prompt and secure closure of the incision is the first goal of the treatment, with gentle repositioning of prolapsed uvea. The surgeon's finger can be used to tamponade the incision site temporarily while sutures are placed. Meanwhile, intravenous acetazolamide (500 mg) and mannitol 20% (1 to 1.5 g/kg) are administered. Once the eye has been closed, the anterior chamber can be reformed through the incision or a paracentesis tract. After this point, a conservative approach probably is appropriate. Some authors advise immediate drainage of the hemorrhage through posterior sclerostomies (usually not possible because it rapidly clots), sometimes combined with a vitrectomy in aphakic patients, especially if the hemorrhage is large. Prognosis for recovery of vision is good as long as the eye can be closed without loss of uvea.
Treatment of postoperative suprachoroidal hemorrhage is directed toward control of the IOP and relief of pain. Most of these eyes do well with this conservative management, and surgical drainage usually is not necessary.8 The indications for drainage include intolerable pain, a persistent flat anterior chamber, and massive “kissing” choroidal detachments (see later). A waiting period of about 7 days after a suprachoroidal hemorrhage is advised for the fibrinolytic response to liquefy the clot and allow for more effective evacuation of the suprachoroidal space. Drainage through a sclerotomy into the suprachoroidal space reveals liquefied blood, which usually is red or black. Occasionally, the fluid drained is a mixture of clear, straw-colored fluid and reddish to black liquefied blood. Bleeding into the vitreous cavity at the time of the hemorrhage and retinal detachment worsen the visual prognosis.
Prevention
Several steps can be taken in high-risk eyes. Before surgery, correction of bleeding problems and discontinuation of inhibitors of platelet aggregation (i.e., acetylsalicylic acid) is recommended. Preoperative intravenous mannitol at the time of surgery should be used. Prophylactic sclerostomies can be helpful. Use of viscoelastic and tight suturing of the scleral flap to prevent hypotony are recommended. The patient is urged to restrict activities (bending, weight lifting) and to avoid Valsalvapositive conditions (constipation, vigorous coughing, sneezing, or nose-blowing) during the early postoperative period.
HYPHEMA
Hyphema is a common postoperative occurrence in glaucomatous eyes after filtration surgery, surgical peripheral iridectomy, and trabeculotomy. Bleeding commonly arises from the ciliary body or cut ends of the Schlemm's canal, although it also might arise from the corneoscleral incision or iris.
In general, hyphema presents at surgery or within the first 2 or 3 days after surgery. Intraoperatively, if a bleeding spot does not stop spontaneously, it must be identified and coagulated. During filtration surgery, bleeding is decreased by performing the internal sclerostomy as far anteriorly as possible.
In most cases, no treatment is necessary and the blood is absorbed within a brief period of time. Cycloplegics, corticosteroids, restriction of activity, and elevation of head of the bed 30° to 45° (to prevent blood from obstructing a superior sclerostomy) are recommended. Increased IOP can occur, particularly if the filtering site is obstructed by a blood clot, and it should be treated if necessary with aqueous suppressants. Injection of tissue-plasminogen activator may be considered (see later). Surgical evacuation is considered depending on the level of IOP, size of hyphema, severity of optic nerve damage, likelihood of corneal blood staining, and presence of sickle trait or sickle cell anemia (infarction of the optic nerve can occur at relatively low IOP, and carbonic anhydrase inhibitors are contraindicated). Liquid blood can be easily removed with irrigation. If a clot has formed, it can be removed by expression with viscoelastic or with a vitrectomy instrument set at low vacuum.
HYPOTONY
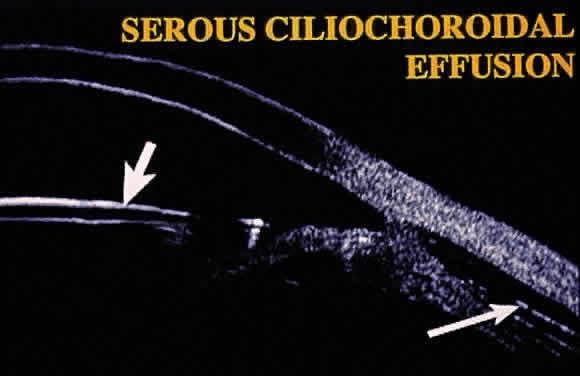
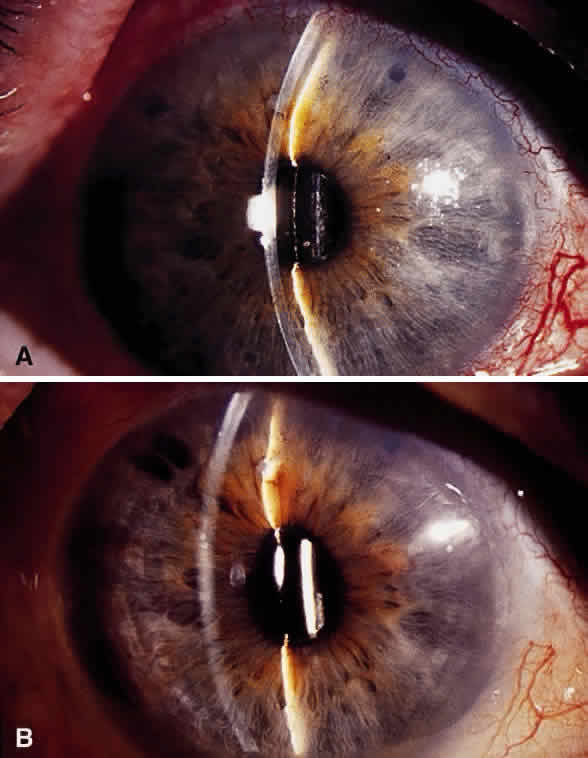
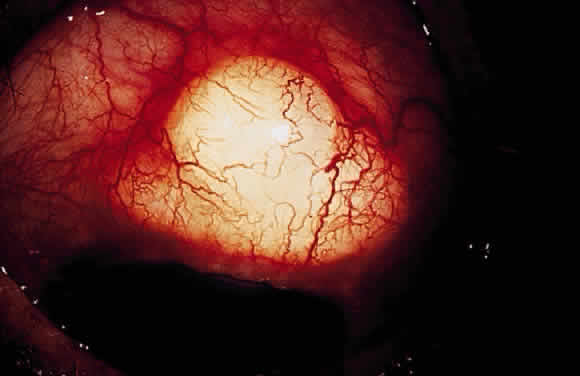
Hypotony (i.e., IOP less than 6 mmHg) after glaucoma surgery can result from excessive aqueous outflow (related to excessive filtration [see later], wound leak, or cyclodialysis cleft) or to reduced aqueous production (related to ciliochoroidal detachment, inflammation, inadvertent use of aqueous suppressants, or extensive cyclodestruction).9 These conditions can coexist. For example, low IOP from overfiltration can induce ciliochoroidal detachment and secondary decreased aqueous production. Possible complications include flat anterior chamber, gradual failure of the bleb, visual loss, cataract, corneal edema, Descemet's membrane folds, choroidal hemorrhage, macular and optic disc edema, and chorioretinal folds (predominantly in young myopic patients). According to Spaeth (Table 1),10 the severity of flat anterior chamber can be classified as grade I when there is peripheral-iris apposition, grade II with pupillary border-corneal apposition, or grade III with lens-corneal touch (see Chapter 15). The central anterior chamber depth also can be described relative to the corneal thickness. Choroidal effusion occurs when fluid collects in the suprachoroidal space (Fig. 2), resulting in forward movement of the lens iris diaphragm with anterior chamber shallowing. On fundus examination, moundlike elevations of the choroid, more commonly in the periphery, are visible.
TABLE 1. Classification of Flat Anterior Chamber
Grade I, peripheral iris-corneal apposition
Grade II, pupillary border-corneal apposition
Grade III, lens-corneal apposition
Hypotony Maculopathy
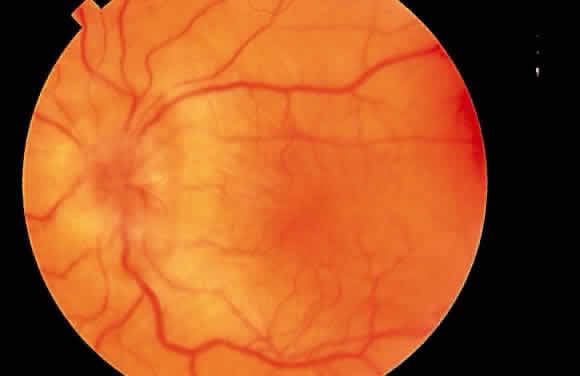
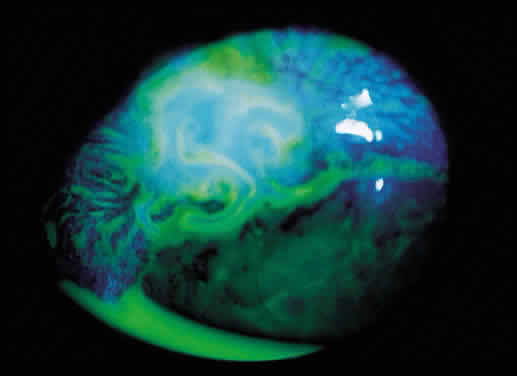
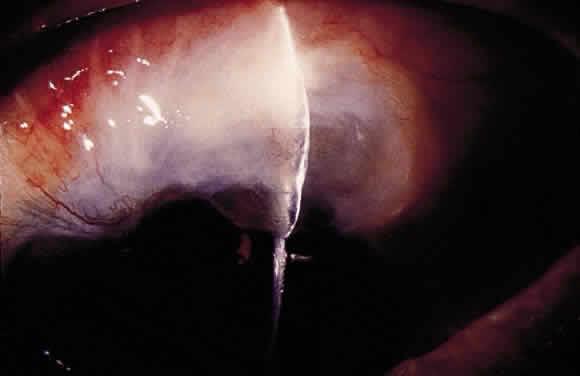
Some patients with intraocular hypotony develop loss of central vision secondary to marked irregular folding of the choroid and retina. Initially, these folds are broad and not sharply delineated. They tend to radiate outward in a branching fashion temporally from the optic disc and concentrically or irregularly nasally to the disc. There may be swelling of the peripapillary choroid simulating papilledema (Fig. 3). The retina often shows a series of stellate folds around the center of the fovea. The retinal vessels are tortuous and sometimes engorged. The primary cause of visual loss is the marked folding of the central retina. Early detection of this condition is important because correction of the cause usually results in visual improvement. In cases of prolonged hypotony, permanent pigmented lines, caused by changes in the retinal pigment epithelium, occur in the macular area and nasally. A postoperative bleb leak and a cyclodialysis cleft were formerly the most common causes of hypotony maculopathy. The incidence of hypotony maculopathy after glaucoma surgery has increased with the use of antifibrotic agents, specifically mitomycin C. A direct toxic of mitomycin cannot be ruled out. The maculopathy is most likely to occur in young myopic patients, who may have a sclera more susceptible to swelling and contraction.11–14
|
Phthisis can occur in some complicated cases with severe chronic hypotony. The sclera shrinks and thickens, and the pull of the extraocular muscles deforms and squares the eyeball. There may be retinal gliosis and formation of a cyclitic membrane in the end stage. Ultimately, impairment of intraocular fluid dynamics results in corneal edema, cataract, and calcification of the corneal epithelium, pigment epithelium, and inner choroid.
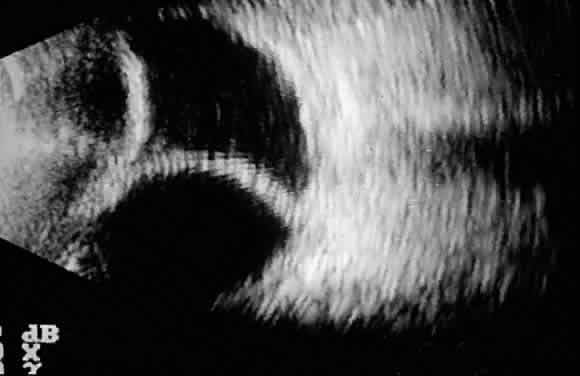
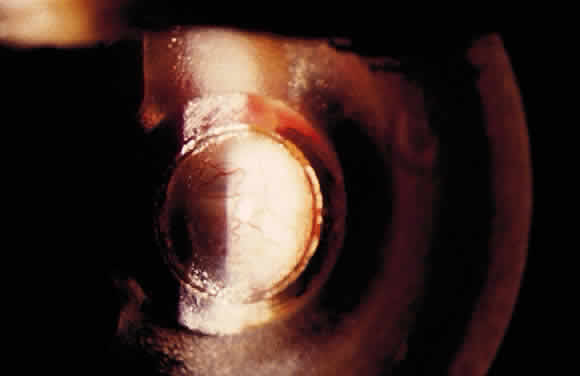
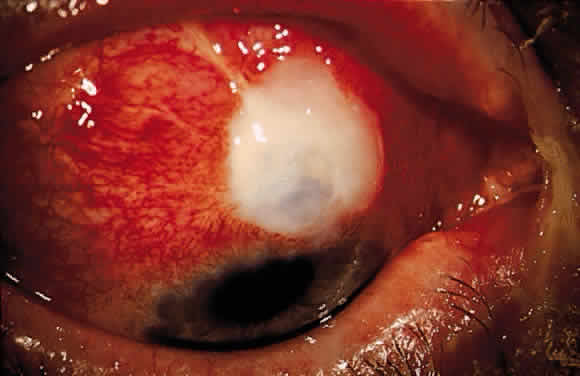
The initial management of early postoperative hypotony with a formed anterior chamber is conservative. Topical steroids and cycloplegics are used. Restrictions in activity (bending, weight lifting) and avoidance of Valsalva-positive conditions are recommended, especially in patients at risk for suprachoroidal hemorrhage (see earlier). If there is hyposecretion related to intraocular inflammation, ciliochoroidal detachment, or both, the initial treatment consists of intense corticosteroid therapy and long-acting cycloplegics, which stabilize the blood-aqueous barrier. Intervention is indicated in patients with hypotony associated with other complications and in persistent, low IOP with loss of visual acuity and hypotony maculopathy. After filtration surgery, prompt management is indicated, also when there is loss of bleb height. Treatment should be aimed at correcting the specific cause of hypotony. When there is lens-corneal touch (flat anterior chamber, grade III) immediate surgical intervention is necessary to prevent endothelial damage and cataract formation (see Chapter 15). Reformation of the anterior chamber with air, balanced salt solution, or, preferably, a viscoelastic can be done at the slit lamp or under the operating microscope through the paracentesis made intraoperatively. Viscoelastic material is best for maintaining, at least temporarily, the anterior chamber depth. When there are large and appositional choroidal effusions, drainage of the fluid also is necessary (Fig. 4). The technique is described in detail in Chapter 15.
When hypotony results from overfiltration of a filtering bleb, several options are available. A large bandage contact lens,15 symblepharon ring,16 and Simmons' shell17 can be helpful. Bandage contact lenses should have a diameter of at least 16 to 17 mm to cover the filtering bleb. They are not efficient in severe and chronic cases. The Simmons' shell is a 22-mm, dome-shaped rigid shell of transparent polymethylmethacrylate. A raised platform on the concave inner surface of the shell is positioned over the sclerostomy site. The curvature is designed to selectively indent the perilimbal area when pressure dressing is applied. The Simmons' shell usually is effective; however, it may be uncomfortable. Tonometry is not possible to monitor the IOP. Decentration of the shell is frequent unless sutured to the conjunctiva. It requires close (daily) monitoring, and corneal complications (epithelial defects and abrasions) are common. It is particularly difficult for monocular patients. Several chemical and thermal treatments have been used to induce an inflammatory reaction in the filtering bleb, which modify the morphologic features of the filtering blebs and increase the IOP. These procedures include topical application of 0.25% to 1% silver nitrate or 50% trichloracetic acid to the bleb surface,18 cryotherapy,19 diathermy and cauterization,20 argon laser,21 and, recently, the neodymium:yttriumaluminum-garnet (Nd:YAG) thermal laser.22 More than one treatment session might be needed to achieve the desired goal. Possible complications include postoperative discomfort, bleb leak, transient increase in IOP, and corneal edema. Cryotherapy is best done under retrobulbar or peribulbar anesthesia. The probe is applied initially to the lateral borders of the bleb. Before starting the freeze, firm pressure is applied with the cryoprobe to bring the bleb surface tissues into apposition with the underlying sclera. Several applications (two to five) using a temperature of -50° to -80°C and a duration of application of 10 to 30 seconds are used. Nd:YAG thermal laser treatment of overfiltering and leaking blebs has been described more recently. It is best done under regional anesthesia. For this procedure, the continuous-wave mode is required. Energy levels range between 3.0 and 4.0 J, with the laser offset between 0.9 and 1.2 mm and the aiming beam focused in the conjunctival epithelium. The goal is to induce whitening and wrinkling of the conjunctival epithelium. A grid pattern of 30 to 40 spots of laser is placed over the entire bleb. Postoperatively, oral aqueous suppressants and a compressive or “torpedo” (i.e., cotton plug placed directly over the bleb surface) patch are used during the first 48 hours. Injection of autologous blood into the bleb can reduce overfiltration and resolve bleb leaks.23–26 Inflammatory cells and serum proteins from the injected blood may accelerate the inflammatory and healing process, which decreases filtration (Fig. 5). Possible complications include hyphema, endophthalmitis, increase in IOP requiring surgical intervention, bleb failure, corneal blood staining, and corneal graft rejection.27–30 Finally, surgical revision may be needed.31–35 Resuturing the scleral flap and scleral patch grafting (when resuturing is not possible) have been successfully used in patients with hypotony maculopathy associated with overfiltering the filtering bleb. Alternatively, two sets of stitches in the scleral flap, with one set tied tightly, can raise the IOP, stretch the sclera, and flatten chorioretinal folds. Sliding conjunctival flaps or free conjunctival grafts also can be helpful (see later).
|
FLAT ANTERIOR CHAMBER AND ELEVATED OR NORMAL INTRAOCULAR PRESSURE
Three conditions should be considered in patients with postoperative flat anterior chamber and elevated or normal IOP: suprachoroidal hemorrhage (see earlier), aqueous misdirection, and pupillary block.
Aqueous Misdirection
Aqueous misdirection, or malignant glaucoma or ciliary block glaucoma, is characterized by a shallowing or flattening of the anterior chamber without pupillary block (i.e., in presence of a patent iridectomy) or choroidal disease (such as suprachoroidal hemorrhage), commonly with an accompanying rise in IOP.36–38 It occurs in 2% to 4% of patients operated on for angle-closure glaucoma but can occur after any type of incisional surgery. The chance of developing malignant glaucoma is greatest in phakic hyperopic (small) eyes with angle-closure glaucoma.
In this condition, aqueous is diverted posteriorly toward the vitreous cavity, increasing the vitreous volume and shallowing the anterior chamber.36 Decompression and shallowing of the anterior chamber appears to be a predisposing factor by inducing forward movement of the peripheral anterior hyaloid. Small choroidal effusions and shallow anterior chamber sometimes occur before the episode of aqueous misdirection. The anterior hyaloid could be placed into direct apposition with portions of the secreting ciliary processes. Thus, aqueous humor might move directly into the vitreous cavity. In hyperopic eyes (with a crowded middle segment), the peripheral anterior hyaloid in its normal position probably is close to the posterior ciliary body. In such eyes, cataract and filtration surgery should be considered as high risk for aqueous misdirection. In aqueous misdirection, a relative resistance to the anterior movement of aqueous humor in the anterior vitreous face or the anterior hyaloid membrane probably occurs. The increased resistance can be related either to abnormal permeability or to available hyaloid surface area for fluid transfer. In normal circumstances, the anterior hyaloid and vitreous offer insignificant resistance to forward fluid flow. In some cases, pupillary block occurs first and is followed by aqueous misdirection. Perhaps a sudden onset of pupillary block forces aqueous humor into the vitreous and expands the vitreous volume, displacing forward the peripheral hyaloid into direct apposition with the ciliary body.39
Aqueous misdirection usually occurs in the early postoperative period after filtration or cataract surgery. The anterior chamber is shallow, and the IOP is high (Fig. 6). However, with a functioning filtration bleb, the IOP may not be high. The peripheral iridectomy is patent, and a dilated examination and B-scan ultrasound confirm the absence of choroidal effusion of hemorrhage. If the adequacy of the surgical iridectomy is in doubt and pupillary block is possible, a laser iridotomy should be performed.
Medical treatment and laser and vitreous surgery all have been useful options to treat aqueous misdirection. This condition is initially managed with mydriatic-cycloplegic drops,40 aqueous suppressants, and hyperosmotics. Topical 1% atropine or 1% cyclopentolate four times daily and 2.5% phenylephrine four times daily are used. These agents hopefully result in a posterior movement of the lens-iris diaphragm. In cases of aphakic aqueous misdirection, mydriatic-cycloplegic drops are of little benefit. However, it is reasonable to use them for their effect on relaxation of the ciliary body muscle. Systemic carbonic anhydrase inhibitors and topical beta-adrenergic blocking agents in full doses are important. Osmotics (isosorbide, glycerin, or intravenous mannitol) also can be helpful to decrease the fluid content of the vitreous cavity and can be given every 12 hours. If it is well tolerated and there are no contraindications, the medical treatment is tried for 2 to 4 days. If the condition is relieved (i.e., the anterior chamber has deepened), the hyperosmostic agents are discontinued first, and the aqueous suppressants are reduced or stopped over several days. Phenylephrine drops can be stopped, but the cycloplegic drops should be continued for months to years or, in some cases, indefinitely to prevent the recurrence of this condition. Medical treatment relieves about 50% of cases of aqueous misdirection. If medical therapy is unsuccessful and the ocular media are clear, a Nd:YAG laser capsulotomy and hyaloidotomy is used to disrupt the anterior vitreous face, especially in pseudophakic and aphakic patients.41 The usual beginning laser energy is between 2 and 4 mJ. The focus is placed posterior to the anterior hyaloid. After a successful Nd:YAG hyaloidotomy, a slight deepening usually is seen, which increases over the next hours. In pseudophakic eyes, a peripheral hyaloidotomy is more efficient than a central hyaloidotomy because the lens capsule and intraocular lens can prevent communication between the vitreous cavity and the anterior chamber. In phakic eyes, Nd:YAG hyaloidotomy can be tried through the peripheral iridectomy, focusing behind the zonules but in front of the ciliary body. However, a clear view and sharp focusing may not be possible, and there is a risk of lens or zonular injury. Pars plana vitrectomy should be considered when other therapies fail.42–44 A standard three-port pars plana vitrectomy, removing the anterior vitreous and part of the anterior hyaloid, is done. In phakic patients, the lens sometimes can be spared, but the probability of recurrence is higher. Pars plana tube shunt insertion with vitrectomy has been recommended to treat patients with aqueous misdirection, especially in patients with angle-closure glaucoma. The implantation of the tube shunt through pars plana can help to prevent recurrence of this condition and can help in long-term control of IOP.44
PREVENTION. In high-risk eyes undergoing cataract or filtration surgery, the decompression and shallowing of the anterior chamber should be minimized. In filtration procedures, the use of viscoelastic and a large peripheral iridectomy can be helpful. Postoperative overfiltration should be avoided with a thick scleral flap sutured tighter and with more sutures than usual. Postoperatively, judicious suture lysis or cutting/pulling releasable sutures and slow tapering of cycloplegics are recommended. A postoperative shallow anterior chamber caused by overfiltration should be vigorously treated.
Pupillary Block
Pupillary block can be caused by adhesions between the iris and lens, pseudophakos, or vitreous. The inability of aqueous humor to pass from the posterior to the anterior chamber results in the forward movement of the peripheral iris and closure of the drainage angle. Pupillary block typically occurs as a flat or shallow anterior chamber with normal or elevated pressure. It may be difficult to distinguish from malignant glaucoma.
Although a peripheral iridectomy is intended at the time of filtration surgery, in a few patients only the stroma of the iris is removed and the posterior pigment epithelium is left intact. In these patients, blockage may develop. In other patients, the iris may become incarcerated in the wound or the iridectomy may be obstructed by intraocular tissue such as Descemet's membrane, anterior hyaloid surface, vitreous (in aphakic eyes), or ciliary processes.
Therapy with cycloplegic-mydriatics may resolve pupillary block, but a Nd:YAG peripheral iridotomy should be done. The anterior chamber readily deepens after iridotomy is performed, although in the presence of localized compartments of blockage, multiple iridotomies are necessary. This deepening usually is associated with the sudden escape of aqueous humor through the iridectomy and confirms the diagnosis of pupillary block. If laser iridotomy cannot be completed, a surgical iridectomy should be done.
VISUAL LOSS
Unexplained loss of central visual field (i.e., “wipeout”) after glaucoma surgery is rare. Older patients with advanced visual field defects affecting the central field with split fixation are at increased risk. Early, undiagnosed postoperative IOP spikes and severe postoperative hypotony have been suspected as causes for wipeout.45,46